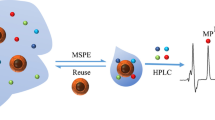
Abstract
A magnetic covalent organic framework (Fe3O4@COF) with core–shell structure was fabricated at room temperature and used as an adsorbent for magnetic solid-phase extraction of polar endocrine-disrupting phenols (4-n-nonylphenol, 4-n-octylphenol, bisphenol A and bisphenol AF). The sorbent was characterized by transmission electron microscopy, FTIR, powder X-ray diffraction and other techniques. The main parameters governing the extraction efficiency were optimized. The phenols were quantified by HPLC with fluorometric detection. The method has attractive features such as low limits of detection (0.08–0.21 ng.mL−1), wide linear ranges (0.5–1000 ng.mL−1), and good repeatability (intra-day: 0.39%–4.99%; inter-day: 1.57%–5.21%). Satisfactory results were obtained when the developed method was applied to determine the four target pollutants in real world drink samples with spiked recoveries over the range of 81.3~118.0%. This indicates that the method is a powerful tool for the enrichment and determination of endocrine-disrupting phenols in drink samples.

A magnetite based covalent organic framework (Fe3O4@COFs) was synthesized with TPAB, TPA and Fe3O4. It was used for magnetic solid-phase extraction of endocrine-disrupting phenols from plastic-packaged tea drink samples coupled with liquid chromatography (LC) for determination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous substances that can interfere with the maintenance of organisms’ balance, reproduction, development, and the synthesis, release, transmission, binding, excretion, action, or removal of hormones [1, 2]. EDCs can be divided into polychlorinated biphenyls, phthalates, phenols, heavy metals, organotins, and other substances based on their structural characteristics [1]. Among them, endocrine-disrupting phenols mainly include 4-n-nonylphenol (NP), 4-n-octylphenol (OP), and bisphenols (BPs), which are widely used as surfactants and plasticizers in the production and synthesis of food packaging materials [3]. These phenols are also widespread in food and the environment [4]. Environmental endocrine disruptors are associated with abnormalities in the reproductive, immune, and nervous systems of humans and animals as well as increased incidence of certain tumors [3].
Several different techniques, such as fluorescent molecular sensing [5], biometrics-chemical analysis methods [6], gas chromatography-mass spectrometrometry (GC–MS) [7], liquid chromatography (LC) [8], and liquid chromatography-mass spectrometrometry (LC/MS–MS) [9], can be used to determine endocrine-disrupting phenols in various samples, but modern chromatographic techniques are commonly used because of their low limits of detection, rapidity, high sensitivity, and convenience. The direct determination of endocrine-disrupting phenols by chromatographic techniques is usually difficult because the concentrations of endocrine-disrupting phenols is becoming lower and lower in different kinds of samples and the sample matrices are very complex. As a consequence, quick, easy, and efficient sample pretreatment techniques are in urgent need for chromatographic analysis [10].
Several sample pretreatment techniques, such as solid-phase extraction (SPE) [7, 9, 11], liquid–liquid extraction [12], soxhlet extraction [13, 14], and solid-phase micro-extraction [15], have been utilized to enrich endocrine-disrupting phenols prior to chromatographic analysis. By introducing magnetic or magnetizable sorbents into the sample solution, magnetic solid-phase extraction (MSPE) has been discovered as a novel sample pretreatment technique for the enrichment and determination of trace pollutants [16]. In MSPE, target analytes are absorbed by magnetic solid sorbents dispersing completely in the sample solution, and magnetic solid sorbents can be rapidly separated from suspensions by using an external magnetic field easily and quickly [17]. MSPE is a prospective method for sample preparations considering its convenience, rapidity, saving expenditure, labor, and spending less time [18]. Studies have focused on exploring and synthesizing novel MSPE adsorbents because of the importance of magnetic adsorbents in the improvement of the extraction efficiency of MSPE for various targets [19]. Various magnetic materials were used to extract trace endocrine-disrupting phenols from foods and other samples, such as magnetic three-dimensional graphene composite [20], Fe3O4@ MIPs [21, 22],, Fe3O4@SiO2@[OMIM] [23], iron-ferric oxide/graphene oxide composite (Fe@Fe2O3/GO) [24] and so on.
Covalent organic frameworks (COFs) are polyporous crystal-structured polymers with building blocks which was linked with covalent bond, and they have been extensively investigated since they were first reported in the year of 2005 [25]. COFs are widely applied as catalysts, gas storage containers, optoelectronics carriers, pretreatment materials for chromatographic separation, chemical sensing sensors and so on because of their good properties, such as tunable pore size, high-porosity, low crystalline density, and super structural stability compared with those of MOFs and other new materials [26,27,28,29,30]. This kind of easily synthesized magnetic covalent organic frameworks which have core–shell structure have been used to achieve the selective enrichment and elimination of peptides from complex biologic samples and they may show great capacity for MSPE adsorbent applications to enrich endocrine-disrupting phenols in food samples [31].
In this work, magnetic covalent organic frameworks which have core–shell structure (Fe3O4@COFs) were synthesized for the selective extraction of four kinds of endocrine-disrupting phenols (4-n-nonylphenol (NP), 4-n-octylphenol (OP), bisphenol A (BPA) and bisphenol AF (BPAF)). And then, we characterized the Fe3O4@COFs microspheres which have a core–shell structure. The feasibility of employing these magnetic covalent organic framework microspheres to be a kind of novel MSPE adsorbent was explored. Four kinds of endocrine-disrupting phenols were chosen as aiming analytes, and HPLC equipped with fluorescence detector was utilized for detection precisely. Groups of single-factor experiments were designated to evaluate the effects of the experimental parameters. The possible mechanism of the Fe3O4@COFs microspheres to extract endocrine-disrupting phenols was also discussed. In short, we established an effective MSPE method which can be utilized for the enrichment and sensitive detection of endocrine-disrupting phenols in plastic-packaged drink samples.
Experimental
Chemicals and reagents
The chemicals and reagents used in this work were as follows: 4-n-Octylphenol (OP) and 4-n-nonylphenol (NP) (Supelco, Bellefonte, PA, USA, www.sigmaaldrich.com); BPA and BPAF (Aladdin Industrial Corporation, Shanghai, China, www.aladdin-e.com) (Table 1); ferric chloride hexahydrate (FeCl3·6H2O), sodium citrate dehydrate (Na3Cit·2H2O), dimethyl sulfoxide (DMSO), tetrahydrofuran, ethylene glycol, acetic acid, HPLC-grade water, ethanol, and molecular sieve type 4A (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, www.sinoreagent.com); 1,3,5-tris(4-aminophenyl)benzene (TAPB) and terephthaldicarboxaldehyde (TPA) (Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China, www.macklin.cn); methanol, acetone, and acetonitrile (Tedia Company, Ohio, USA, www.tedia.com); and dichloromethane and n-hexane (Concord Technology, Tianjin, China, tjconcord.labscn.com.cn). Reagents and chemicals at least analytical grade were used in this work. Stock solution containing BPA, BPAF, OP, and NP at 1 mg·mL−1 was prepared by dissolving the four kinds of endocrine-disrupting phenols in a 25 mL volumetric flask. The stock solution was diluted gradually using methanol to obtain a series of standard solutions with different concentration, and all of the solutions were placed in the dark at 4 °C prior to use.
Synthesis of Fe3O4 and Fe3O4@COFs
Fe3O4 and Fe3O4@COFs were synthesized refer to the method in reference [31] with slight modifications. The specific details about synthesis are given in the Electronic Supporting Material.
Instruments
A Shimadzu LC-20A HPLC (Shimadzu Scientific, Japan) furnished with an RF-20A fluorescence detector (FLD) (Shimadzu Scientific, Japan) was used for the analysis. Chromatographic separation was conducted on an Agilent Zorbax Eclipse XDB-C8 column (150 mm × 4.6 mm, 5 μm) under the column oven temperature of 35 °C. 1 mL·min−1 was set as the experimental flow rate. 20 μL was set as the injection volume, and the HPLC-grade water (A) and acetonitrile (B) were used as the mobile phases of this LC detection method. The following was the gradient elution of this method: (1) 0 min, 40% B; (2) 24 min, 80% B; (3) 28 min, 80%B; (4) 28.2 min, 45% B, and (5) 30 min, 45% B. The following parameters were the settings of FLD: 220 nm excitation and 315 nm emission.
The images of Fe3O4 and Fe3O4@COFs microspheres were got using a JEOL model JEM-2010 (HR) transmission electron microscope (TEM; Tokyo, Japan). The Cu-Kα radiation (γ = 1.5478 Å) mode of the D/max-Rb diffractometer (Rigaku, Japan) was used to achieve X-ray diffraction (XRD) measurements and the angular measurement was ranged from 10° to 80°. Small angle XRD measurements were got using Ultima IV mode of the D/max-Rb diffractometer (Rigaku, Japan). SQUID-VSM (Quantum Design, San Diego, CA, USA) was used to measure the magnetic properties of these two kinds of synthesized microspheres at 25 °C. Nicolet 710 IR infrared spectrometer (Thermo Scientific, Waltham, MA, USA) was used to obtain the data of the Fourier transform infrared (FT-IR) spectra. N2 (99.995%) on Fe3O4 and Fe3O4@COFs was subjected to gas adsorption–desorption measurements on a Micromeritics ASAP 2020 surface area and pore size analyzer (Norcross, GA, USA).
MSPE process
For the MSPE of the four kinds of phenols, 25 mL of the sample solution was added in a glass bottle, and 40 mg of Fe3O4@COFs microspheres was added to the solution. The mixture solution was shaken at 300 times min−1 in a water bath chader for 30 min at room temperature. The sorbents were separated from the aqueous phase with the assist of the external magnet located outside the glass vial, and the supernatant was discarded entirely. Subsequently, 3 mL addition of methanol was put into the isolated MSPE sorbents, and the analytes were eluted by sonicating for 3 min and vortexing for 3 min. Then, this operation was repeated again to ensure the process of desorption is complete. The eluted solution was mixed and dried under N2 at 35 °C. Finally, the residue was redissolved using 250 μL of methanol. The redissolved samples were injected to LC, and the recoveries were considered to assess the enrichment efficiency of the four kinds of endocrine-disrupting phenols. The used Fe3O4@COFs microspheres were ultrasonically washed using methanol thrice prior to their next use.
Sample collection
In this work, four kinds of different plastic-packaged tea drinks from four different manufacturers were used as the real samples. These four kinds of tea drinks were as follows: ice black tea from Uni-President China Holdings Ltd. (Taiwan, China), jasmine tea from Master Kong Holdings Ltd. (Tianjin, China), greengage green tea from Dali Foods Group (Quanzhou, Fujian, China), chapai peach oolong tea from Nongfu Spring Co., Ltd. (Hangzhou, Zhejiang, China). These four kinds of tea drinks were purchased from a local supermarket (Jinan, China), and then they were centrifuged for 10 min under the condition of 4 °C and at the speed of 10,000 rpm (9×g) to get rid of the precipitate. Besides, to ensure the clarity of the supernatants and prevent the blocking of the injector, they were filtered through micropore membranes which were 0.22 μm. The processed tea drink samples were reserved in clean glass bottles colored brown under the temperature of 4 °C for the following MSPE experiments.
Before the MSPE process, 5 mL of processed tea drink samples was diluted to 25 mL with ultrapure water, and then the pH of the diluted samples was adjusted using 1 mol·L−1 NaOH or 1 mol·L−1 HCl.
Results and discussion
Choice of materials
Numerous materials, such as Fe3O4@MIPs [21, 22], and Fe3O4@SiO2@[OMIM] [24], Fe3O4@COF(TpBD) [32] can be used as MSPE adsorbents. Fe3O4@COFs is a kind of novel easily synthesized magnetic COFs. It can be synthesized at room temperature in 15 min, which is much shorter than the synthesis time of the material synthesized in the analogous article (more than 12 h) (the time does not include the time of Fe3O4 preparation) [32]. The new material possesses optimal properties, such as high surface area (178.87 m2·g−1), numerous specific adsorption sites, large pore volume (0.43 cm3·g−1), and numerous surface functional groups (C=N, –COOH, –NH2, and so on) [31]. These functional groups may form π interactions, hydrogen bonds and other acting forces with target sites on the molecules, which make them appropriate as candidate adsorbents of MSPE to enrich target pollutants from various samples.
Characterization of Fe3O4@COFs
The high-magnification morphologies of the prepared Fe3O4 and Fe3O4@COFs microspheres were observed under a TEM (Fig. 1a and b). Both of the images show that the bare Fe3O4 core was nearly spherical and its average diameter was 150–250 nm. A typical core–shell structure made up of a Fe3O4 core and a COF shell was formed and the thickness of the COF shell was 25 nm approximately.
a TEM images of Fe3O4 microspheres at 25,000× magnification; b TEM images of Fe3O4@COFs microspheres at 25,000× magnification; c FT-IR spectra of TAPB, TPA, Fe3O4, and Fe3O4@COFs; d XRD patterns of Fe3O4 and Fe3O4@COFs microspheres in air; e FT-IR spectra of the Fe3O4@COFs microspheres in air, in MA, in 1 mol L−1 HCl for 24 h, in 1 mol L−1 NaOH for 24 h, and in water for 24 h; f magnetization curves of Fe3O4 and Fe3O4@COFs microspheres; (G1 and 2) N2 adsorption–desorption isotherm and pore size distribution of Fe3O4@COFs microspheres
FT-IR was performed to determine the specific functional groups on the Fe3O4@COFs microspheres and the successful synthesis of them (Fig. 1c). A typical absorption band at 585 cm−1 was assigned to the Fe–O–Fe vibration, and the absorption bands at 3421, 1640, and 1388 cm−1 indicated the existence of carboxyl groups. In comparison with the bare Fe3O4 core and the TAPB and TPA monomers, the prepared Fe3O4@COFs exhibited new characteristic peaks at 1450–1600 and 1610 cm−1, which were ascribed to the benzene skeleton and C=N vibrations of the COF material, respectively. The FT-IR data were consistent with previous findings [31], which suggested the successful formation of the COF shell through the process of condensation polymerization.
XRD (Fig. 1d) was performed to confirm the crystalline structure and phase purity of Fe3O4 with and without the COF shell. The positions of all of the diffraction peaks at 2.7°, 19.5°, 31°, 35.5°, 43.5°, 57.5°, and 62.5° for Fe3O4 and Fe3O4@COFs confirmed that the products had great crystal structure and the XRD pattern of the synthesized Fe3O4@COFs was consistent with previous observations, implying the successful synthesis of the material [31]. The XRD of Fe3O4 was compared with Fe3O4@COFs, and the XRD of the Fe3O4@COFs suggested broad diffraction peaks at approximately 2.7 ° and 19.5 °, which might be ascribed to the lower crystallinity after the coating of the COF shell.
The chemical stability of Fe3O4@COFs microspheres in organic solvents and aqueous solutions with different pH values remarkably affects the process of adsorption and elution as well as confirms if a novel material is a suitable MSPE sorbent. Therefore, their chemical stability is important. In Fig. 1e, there are no distinct changes in the FT-IR spectra were discovered in aqueous solutions with 1 mol·L−1 NaOH and 1 mol·L−1 HCl, as well as organic solvents, indicating that Fe3O4@COF microspheres were stable under different experimental conditions apparently because of the strong C=N covalent bond in polyimine-linkage skeletons. Therefore, Fe3O4@COFs microspheres can work as a suitable MSPE adsorbent.
The magnetization curves of Fe3O4 and Fe3O4@COFs microspheres are shown in Fig. 1f. Hysteresis, coercivity, or remanence was not found in the magnetization process of the two kinds of synthesized microspheres. The two microspheres exhibited a super paramagnetic characters, and the Fe3O4@COFs possessed a saturated magnetization value of 62.5 emu·g−1, although this value slightly decreased compared with that of the bare Fe3O4 (81.5 emu·g−1). Fe3O4@COFs was sensitive to an external magnetic field owing to such high saturation magnetization. In the inset of Fig. 1f, Fe3O4@COFs achieved excellent dispersion in water, thereby forming a brown suspension that was uniform overnight without precipitation. However, with the help of an external magnet, Fe3O4@COFs was well collected (~1 min) from their uniform dispersion solution. Then, the dispersion became transparent.
As are shown in Fig. 1g and h, the Brunauer–Emmett–Teller surface area of Fe3O4@COFs calculated from the N2 sorption isotherm was 166.5 m2·g−1, and the volume of its pore was 0.26 cm3·g−1. And the values for bare Fe3O4 were 10.7 m2·g−1 and 0.11 cm3·g−1, respectively, which were much lower than those of Fe3O4@COFs. By this token, Fe3O4@COFs were more suitable as magnetic solid-phase sorbents than bare Fe3O4 because of their large pore volume (0.26 cm3·g−1) and large surface area (166.5 m2·g−1).
Optimization of method
The following parameters were optimized: (a) eluent; (b) amount of sorbent; (c) sample pH value; (d) extraction time; (e) ionic strength; (f) volume of eluent. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were able to achieve the best results: (a) volume of MA, 6 mL (3 mL × 2); (b) pH of the sample, 6; (c) amount of Fe3O4@COFs, 40 mg; (d) NaCl, 0%; (e) extraction time, 30 min.
Possible extraction mechanisms of Fe3O4@COFs for endocrine-disrupting phenols
The physicochemical properties of the four kinds of endocrine-disrupting phenols obtained with the optimal conditions are shown in Table 1. The MSPE performance was excellent because of the following reasons: Firstly, the hydrogen bonding between the hydroxyl groups of the endocrine-disrupting phenols and the amino groups as well as the C=N group on the surface of the Fe3O4@COFs microspheres might improve the extraction efficiency. Besides, the hydrophilicity and polarity of the particles improved because of the existence of the amino groups on Fe3O4@COFs, resulting in their suitability for the extraction of these four kinds of endocrine-disrupting phenols. Secondly, the large logKow values of the endocrine-disrupting phenols indicated that they have a large tendency to escape from the water phase to the surface of the Fe3O4@COF microspheres. And the large pKa values of the endocrine-disrupting phenols further contributed to this tendency. Thirdly, π interaction may exist among the benzene rings of the endocrine-disrupting phenols and the benzene rings of the COF shell coating on the Fe3O4@COFs microspheres and then as a result, the adsorption phenomena possibly occurred. Fourthly, as a kind of magnetic COF material, Fe3O4@COFs has an indicative mesoporous feature owing to the typical type IV characteristics which was presented [31]. The Brunauer–Emmett–Teller (BET) surface areas of Fe3O4@COFs calculated from the N2 sorption isotherm were 166.5 m2·g−1, and the volume of its pore was 0.26 cm3·g−1. Fe3O4@COFs was considered as an adsorbent with good performance as a result of their large external surface area and mesoporous structure. In summary, the hydrophobicity and ionization of the endocrine-disrupting phenols were essential for the remarkable adsorption performance of Fe3O4@COFs for the analytes. In addition to hydrogen bonding, the π stacking interaction between endocrine-disrupting phenols and Fe3O4@COF microspheres as well as the mesoporous structure of Fe3O4@COFs also played important roles.
Method evaluation
The analytical data under the optimal conditions are summarized in Table 2 and Table 3. As is demonstrated in Table 2, the recoveries for endocrine-disrupting phenols were much higher than other pollutants under the optimal experimental conditions respectively, so that Fe3O4@COFs absorbs endocrine-disrupting phenols selectively and can get satisfying results. And as shown in Table 3, within the concentration range of 0.05–1000 ng·mL−1, the optimized MSPE displayed excellent linearity (R ≥ 0.995). The limits of detection (LODs) which was based on signal-to-noise (S/N) ratios of 3 was within the scope of 0.08 ng·mL−1 to 0.21 ng·mL−1. The limits of quantification (LOQs) calculated by the S/N ratios of 10 was in the range of 0.39 ng·mL−1 to 0.85 ng·mL−1. The relative standard deviations (RSDs) of interday (n = 5) and intraday (n = 5) assays were in the range of 1.57%–5.21% and 0.39%–4.99% respectively for the four kinds of endocrine-disrupting phenols. In Table 4, the LODs of the method were much lower than those of else methods, and the linear range of this method was wider than others [21, 22, 33,34,35,36] for endocrine-disrupting phenols. The Fe3O4@COFs microspheres can be used more than 20 times repeatedly without loss of enrichment efficiency. These experimental data showed that Fe3O4@COFs were novel extraction adsorbents, which were adaptable for the extraction of endocrine-disrupting phenols in MSPE.
Analysis of real drink samples
Four different kinds of plastic-packaged tea drinks were analyzed under optimized conditions to verify the practicability of the recommended method. Recoveries of the real samples were obtained via adding the standard solutions of endocrine-disrupting phenols at concentrations of 5, 50, and 100 ng·mL−1. In Table 5, no endocrine-disrupting phenols were detected in the four plastic-packaged tea drinks, and the recoveries were between 81.3% and 118.0% with RSDs (0.1%–8.3%). These results revealed that different drink matrices hardly influenced this novel MSPE. Therefore, the method with convenience and competitive sensitivity was precise for the enrichment and determination of the target endocrine-disrupting phenols in real plastic-packaging drink samples. The typical chromatograms of real tea drink samples are displayed in Fig. 2. Conclusion can be drawn on the basis of these results that novel MSPE method can be used to determine four kinds of endocrine-disrupting phenols in real drink samples.
Conclusion
The purpose of this paper was to investigate the possibility of magnetic covalent organic framework to enrich endocrine-disrupting phenols with high enrichment factor in environmental water systems. This paper had described a novel, rapid, convenient and sensitive determination method of endocrine-disrupting phenols. This method earned good linear range, reproducibility and low detection limit at several ng mL−1 level for OP, NP, BPA and BPAF. These facts demonstrated that magnetic COFs were of great potential to be applied as adsorbent for MSPE of endocrine-disrupting phenols in plastic-packaged food, drinks and environmental waters. It is expected that magnetic COFs may be a kind of efficient MSPE adsorbents for monitoring many other pollutants in food due to its merits exhibited in this work.
References
Zeng BW, Jiang P (2005) Environmental hormone. Chemical Industry Press, Beijing, pp 1–15
Du HF, Yan HF (2005) Progress on detection and analysis method of endocrine disrupting compounds. J Hyg Res 34:493–496
Jin F, Zhang Y, Wang J (2008) Review on advance of phenolic endocrine disrupting chemicals in food. Sci Technol Food Ind 29:263–270
Guenther K, Heinke V, Thiele B, Kleist E, Prast H, Raecker T (2002) Endocrine disrupting nonylphenols are ubiquitous in food. Environ Sci Technol 36:1676–1680
Del Olmo M, Zafra A, Gonzalez-casado A, Vilchez JL (1998) The use of β-cyclodextrin inclusion complexes for the analysis of bisphenol a residues in water by spectrofluorimetry. Int J Environ An Ch 69:99–110
Seifert M, Brenner-Wei β G, Haindl S, Nusser M, Obst U, Hock B (1999) A new concept for the bioeffects-related analysis of xenoestrogens: hyphenation of receptor assays with LC-MS. Fresen J Anal Chem 363:767–770
Mol HGJ, Sunarto S, Steijger OM (2000) Determination of endocrine disruptors in water after derivatization with N-methyl-N-(tert-butyldimethyltrifluoroacetamide) using gas chromatography with mass spectrometric detection. J Chromatogr A 879:97–112
Konig H, Ryschka R, Strobel W (1985) Trennung, identifizierung und bestimmung nichtionischer tenside mittels hochleistungs-fliissigkeits-chromatographie. Z Anal Chem 321:263–267
Tran NH, Hu JY, Ong SL (2013) Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC–MS/MS and isotope dilution. Talanta 113:82–92
Gong SX, Wang XL, Liu W, Wang ML, Wang X, Wang ZW, Zhao RS (2017) Aminosilanized magnetic carbon microspheres for the magnetic solid-phase extraction of bisphenol A, bisphenol AF, and tetrabromobisphenol A from environmental water samples. J Sep Sci 40:1755–1764
Miyakoda H, Tabata M, Onodera S, Takeda K (1999) Passage of bisphenol A into the fetus of the pregnant rat. J Health Sci 45:318–323
Gonzalez-Casado A, Navas N, Del Olmo M, Vilchez JL (1998) Determination of bisphenol A in water by micro liquid-liquid extraction followed by silylation and gas chromatography-mass spectrometry analysis. J Chromatogr Sci 36:565–569
Marcomini A, Giger W (1987) Simultaneous determination of linear alkylbenzenesulfonates, alkylphenol polyethoxylates, and nonylphenol by high performance liquid chromatography. Anal Chem 59:1709–1715
Chalaux N, Bayona JM, Albaiges J (1999) Determination of nonylphenols as pentafluorobenzyl derivatives by capillary gas chromatography with electroncapture and mass spectrometric detection in environmental matrices. J Chromatogr A 686:275–281
Salafranca J, Batlle R, Nerin C (1999) Use of solid-phase microextraction for the analysis of bisphenol A and bisphenol A diglycidyl ether in food simulants. J Chromatogr A 864:137–144
Zhao Q, Lu Q, Feng YQ (2013) Dispersive microextraction based on magnetic polypyrrole nanowires for the fast determination of pesticide residues in beverage and environmental water samples. Anal Bioanal Chem 405:4765–4776
Xiao DL, Lu T, Zeng R, Bi YP (2016) Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183:2655–2675
Zou Y, Chen YZ, Yan ZH, Chen CY, Wang JP, Yao SZ (2013) Magnetic solid-phase extraction based on tetrabenzyl modified Fe3O4 nanoparticles for the analysis of trace polycyclic aromatic hydrocarbons in environmental water samples. Analyst 138:5904–5912
Huo SH, Yan XP (2012) Facile magnetization of metal–organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 137:3445–3451
Liu L, Feng T, Wang C, Wu QH, Wang Z (2014) Magnetic three-dimensional graphene nanoparticles for the preconcentration of endocrine-disrupting phenols. Microchim Acta 181:1249–1255
Xu Z, Ding L, Long YJ, Xu LG, Wang LB, Xu CL (2011) Preparation and evaluation of superparamagnetic surface molecularly imprinted polymer nanoparticles for selective extraction of bisphenol a in packed food. Anal Methods 3:1737–1744
Wu XQ, Wang XY, Lu WH, Wang XR, Li JH, You HY, Xiong H, Chen LX (2016) Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J Chromatogr A 1435:30–38
Chen SQ, Chen JP, Zhu SX (2016) Solid phase extraction of bisphenol a using magnetic core–shell (Fe3O4@SiO2) nanoparticles coated with an ionic liquid, and its quantitation by HPLC. Microchim Acta 183:1315–1321
Li F, Cai CC, Cheng J, Zhou HB, Ding KR, Zhang LZ (2015) Extraction of endocrine-disrupting phenols with iron-ferric oxide core–shell nanowires on graphene oxide nanosheets, followed by their determination by HPLC. Microchim Acta 182:2503–2511
Coˇte AP, Benin AI, Ockwig NW, Keeffe MO, Matzger AJ, Yaghi OM (2005) Porous, crystalline, covalent organic frameworks. Science 310:1166–1170
Wang X, Han X, Zhang J, Wu XW, Liu Y, Cui Y (2016) Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J Am Chem Soc 138:12332–12335
Huang N, Chen X, Krishna R, Jiang DL (2015) Two-dimensional covalent organic frameworks for carbon dioxide capture through Channel-Wall functionalization. Angew Chem Int Ed 54:2986–2990
Stange P, Fumino K, Kudwig R (2013) Ion speciation of protic ionic liquids in water: transition from contact to solvent-separated ion pairs. Angew Chem Int Ed 52:2990–2994
Yang CX, Liu C, Cao YM, Yan XP (2015) Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem Commun 51:12254–12257
Li Z, Zhang YW, Xia H, Mu Y, Liu X (2016) A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu(2+) ions. Chem Commun 52:6613–6616
Lin G, Gao CH, Zheng Q, Lei ZX, Geng HJ, Lin Z, Yanga HH, Cai ZW (2017) Room-temperature synthesis of core–shell structured magnetic covalent organic framework for efficient enrichment of peptides and simultaneous exclusion of proteins. Chem Commun 53:3649–3652
Li N, Wu D, Hu N, Fan GS, Li XT, Sun J, Chen XF, Suo YR, Li GL, Wu YN (2018) Effective enrichment and detection of trace polycyclic aromatic hydrocarbons in food samples based on magnetic covalent organic framework hybrid microspheres. J Agric Food Chem 66:3572–3580
Wang XY, Deng CH (2015) Preparation of magnetic graphene @polydopamine@Zr-MOF material for the extraction and analysis of bisphenols in water samples. Talanta 144:1329–1335
Yang JJ, Li Y, Wang JC, Sun XL, Cao R, Sun H, Huang CN, Chen JP (2015) Molecularly imprinted polymer microspheres prepared by Pickering emulsion polymerization for selective solid-phase extraction of eight bisphenols from human urine samples. Anal Chim Acta 872:35–45
Herrero-Hernández E, Carabias-Martínez R, Rodríguez-Gonzalo E (2009) Use of a bisphenol-A imprinted polymer as a selective sorbent for the determination of phenols and phenoxyacids in honey by liquid chromatography with diode array and tandem mass spectrometric detection. Anal Chim Acta 650:195–201
Maragou NC, Lampi EN, Thomaidis NS, Koupparis MA (2006) Determination of bisphenol A in milk by solid phase extraction and liquid chromatography–mass spectrometry. J Chromatogr A 1129:165–173
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21777089), the Natural Science Foundation of Shandong Province (ZR2018MB040), the Key Research and Development Program of Shandong Province (2017GSF17107 and 2018GSF117036), Shandong Provincial Key Laboratory of Test Technology for Material Chemical Safety (2018SDCLHX001), and the Shandong Province Taishan Scholar Program (ts201712063).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1310 kb)
Rights and permissions
About this article
Cite this article
Deng, ZH., Wang, X., Wang, XL. et al. A core-shell structured magnetic covalent organic framework (type Fe3O4@COF) as a sorbent for solid-phase extraction of endocrine-disrupting phenols prior to their quantitation by HPLC. Microchim Acta 186, 108 (2019). https://doi.org/10.1007/s00604-018-3198-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3198-3