Abstract
A colorimetric assay is described for highly selective and sensitive determination of Hg(II) ions by using gold nanoparticles (AuNPs) functionalized with dithioerythritol (DETL). This method relies on the unique optical properties of DETL-functionalized AuNPs as well as the thiophilicity of both AuNPs and Hg(II). In the presence of DETL, the AuNPs aggregate due to ligand exchange between thiol groups of DETL and the citrate ions on the surface of AuNPs. This induces a color change from red to blue. On addition of Hg(II), the thiol groups preferably interact with Hg(II) rather than with AuNPs. Thus, the DETL is released from the surface of the AuNPs and binds to Hg(II). This triggers the redispersion of the AuNPs. The ratio of absorbances at 650 and 525 nm drops linearly in two Hg(II) concentration ranges (viz. from 0.1 to 0.5 μM, and from 0.5 to 5 μM). The ions Cu(II), Pb(II), and Cd(II) do not interfere even in the absence of masking agents. The detection limit is as low as 24 nM.

A highly selective colorimetric method based on gold nanoparticles via double ligand exchange reaction is described for determination of Hg2+. This assay can selective detect Hg2+ with no response to major interfering metal ions such as Cu2+, Pb2+, and Cd2+ in the absence of masking agents compared with previous works.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg2+) is one of the most stable forms of mercury contamination [1]. Hg2+ displays strong affinity for the ligands containing sulfur atoms and induces the obstructing of sulfhydryl groups of membranes, enzymes, and proteins [2]. Hg2+ is also related to the abnormal functions of gastrointestinal tract, brain, kidney, and liver [3]. Owing to its serious hazardous effects to human safety and health, there is an urgent demand to develop a highly selective and sensitive method for determination of Hg2+ in aqueous media [4].
Methods such as inductively coupled plasma mass spectrometry [5], atomic absorption spectroscopy [6], and atomic fluorescence spectroscopy [7] etc., have been found to detect Hg2+. Moreover, a great effort has been exerted to develop Hg2+ detection methods using fluorophores [8], organic chromophores [9], DNAzymes [10], oligonucleotides [11], and colorimetric methods [3, 4]. But there are still major disadvantages associated with some of these methods regarding their practical application: poor repeatability, sophisticated synthesis of probe materials and solubility in aqueous media.
Because their absorption coefficients are several orders of magnitude higher than those of common organic dyes in the visible region, gold nanoparticals (AuNPs) have been reported as a class of important colorimetric materials [12]. Simultaneously, the surface of AuNPs is easily modified by some ligands such as -SH, -NH2 etc.. This can enhance the selectivity and sensitivity of AuNP-based colorimetric methods, and expands its application [13]. When the aggregation or redispersion of AuNPs occurs, the color turns blue or purple or returns red. This is because the nanoparticles coming in close proximity or separating to each other inducing the coupling of the plasmon absorbance. Employing this concept, researchers reported AuNP-based colorimetric assays for the determination of DNA [14], antibiotics [7, 13], and metal ions [15]. As for metal ion assays, the functional groups on AuNPs shows a critical role in the recognition properties. The one of the important tools to introduce the custom functionality on AuNPs is ligand exchange reaction. 4-mercaptophenylboronic acid [16], 4-amino-3-hydrazino-5-mercapto-1,2,4-triazol [17], and 6-Thioguanine [18] are used as capping reagents of AuNPs through ligand exchange reactions for the detection of Hg2+, dopamine, and Hg2+, respectively.
Comparing with major competing heavy metal ions such as Cd2+ and Pb2+, Hg2+ shows stronger thiophilic tendency [18]. Therefore, many sulfur-based functional groups of chromophores and fluorescent receptors have selected for the selective determination of Hg2+ [19]. Inspired by these observations, Kim et al. designed a highly sensitive AuNP-based colorimetric detecting of Hg2+ through ligand exchange reaction [20]. With the addition of Hg2+, the DETL modified AuNPs can quickly aggregate through specific sulfur-Hg2+-sulfur interaction. However, this novel method is interfered by other metal ions including Cu2+ and Ba2+. It needs masking agent ethylenediamine tetraacetic acid (EDTA) to eliminate interference. Moreover, Chen et al. designed a non-aggregation colorimetric detection for Hg2+ based on AuNPs and thiocyanuric acid (TGA) [21]. The AuNPs occurs aggregation due to the formation of Au-S bonds between three thiol groups of TGA and AuNPs. With the addition of Hg2+, thiol group is more tend to interact with Hg2+ than AuNPs which induces AuNPs redispersion again. This non-aggregation method shows highly selectivity toward Hg2+ in the absence of masking agents [18]. According to those observations, we guess whether DETL might be used for the modification of AuNPs just like the TGA, and thus improve the selectivity. Fortunately, Tsai et al. reported a research about the controlled formation and characterization of DETL conjugated AuNPs [22]. The DETL conjugate with AuNPs can form cross-linking mode by control the concentration of DETL and AuNPs, and thus induce the aggregation of AuNPs. Based on the previous researches, we herein report an attractive highly selective nanoprobe for Hg2+ detection through twice ligand exchange reaction: (i) Au-S reaction exchange the citrate ions of surface of AuNPs; and (ii) specific Hg2+-sulfur reaction exchange the thiol groups on the surface of dithioerythritol-modified AuNPs (DETL-AuNPs). This work is based on the formation of Au-S bonds between DETL and AuNPs, and higher affinity of DETL toward Hg2+ over AuNPs; such an idea has been attempted in this work for the development of Hg2+ nanoprobe in aqueous media. The designed colorimetric method shows highly selectivity toward Hg2+ in the absence of masking agents compared with previous works [20]. Hg2+ results in colorimetric change because of the change in SPR absorption.
Experimental section
Chemicals
Chloroauric acid hydrated (HAuCl4·4H2O), trisodium citrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (www.sinopharmholding.com, Shanghai, China). Dithioerythritol was purchased from TCL (www.macklin.cn, Shanghai, China). Mercuric nitrate was purchased from Xiya Chemical Industry Co., Ltd. (www.xiyashiji.com, Shandong, China). All the other chemicals were of analytical grade and used without further purification. Each experimental process was using ultrapure water (18.2 MΩ·cm, Milli-Q Millipore). All of the aqueous solutions were prepared with ultrapure water.
Apparatus
Colorimetric measurement was recorded by a UV–2700 spectrophotometer (www.shimadzu.com, Shimadzu corporation, Kyoto, Japan) to obtain the absorption spectra of reaction solution using a quartz cuvette (1-cm pathlength), and the transmission electron microscopy (TEM) images were performed on a TECNAI T20 G2 electron microscope instrument operated at an accelerating voltage of 200 KV (www.antpedia.com, FEI, Netherland). The concentration of Hg2+ in the real samples was measured using atomic absorption spectroscopy (AAS).
Preparation of functionalized AuNPs with DETL
All glassware was soaked in a bath of freshly prepared aqua regia for 30 min, thoroughly washed with ultrapure water (18.2 MΩ·cm, Milli-Q Millipore), and finally dried in constant temperature oven before use.
AuNPs were prepared by citrate-mediated reduction of HAuCl4 according to reported method with a bit modification [7, 15] (the detailed information please see in Electronic Supporting Material). The concentration of the prepared AuNPs was about 8.22 nM according to Beer-Lambert’s low with an extinction coefficient of 2.78 × 108 M−1 cm−1 at 520 nm for the 13-nm AuNPs [7].
DETL-AuNPs were prepared by adding a certain amount of DETL into AuNPs solution [22] (the detailed information please see in Electronic Supporting Material).
Colorimetric detection of Hg2+
Colorimetric detection of Hg2+ is evaluated by detecting a range of concentration of Hg2+ with the DETL-AuNPs system. The pH value was adjusted to 6.6, and then a series of Hg2+ solutions (0.01 mL) with different concentrations (0, 0.1, 0.25, 0.35, 0.45, 0.5, 1, 2, 3, 5, 10 μM) was added into the DETL-AuNPs solution. After thorough shaking, the color change of the mixture was observed and recorded. 15 min later, the UV-vis absorption spectra of resulting solution was measured by the UV-vis spectrophotometer over the wavelength range from 200 nm to 800 nm. During the detection process, the concentration of AuNPs was 2.12 nM. All assays were performed at 30 °C.
Analysis of real samples
Analysis of real samples is used to test the applicability of DETL-AuNPs in detecting of Hg2+. Samples of river water, tap water and pond water were collected from the Hsiang River (Changsha, China), our laboratory and Lake of Peach (Changsha, China), respectively. Firstly, the water samples were filtered through a 0.22 μm syringe filter. And then, we adjusted the pH of the samples to 6.6. Finally, the samples were prepared by spiking with the standard solutions of Hg2+. The final concentration was 0.2, 0.5 and 4 μM, respectively.
Results and discussion
Mechanism of the colorimetric assay
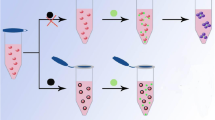
Scheme 1 shows the mechanism for the determination of Hg2+. The as-prepared AuNPs are stable in aqueous solution, due to the electrostatic repulsion of the negative charge of citrate ions prevents the aggregation of AuNPs. Moreover, the AuNPs solution shows wine-red color, because suitable visible light induces the collective oscillations of the surface electrons that is highly depended on the interparticle distance. With the addition of DETL, the thiol group of DETL can be quickly and easily attached to the surface of the AuNPs through ligand exchange of thiol group and citrate ions, and formation of Au-S bonds. Accordingly, the AuNPs can be cross-linked together because of the two thiol groups of DETL, and the solution color changes to blue [22]. While the DETL-AuNPs solution are added with Hg2+, the thiol groups leave the surface of AuNPs and combine with Hg2+ formation of Hg2+-sulfur bond because thiol groups are more tend to interact with Hg2+ than AuNPs. When thiol groups leave AuNPs, the citrate ions ligand exchange with thiol groups, then citrate ions attach to the surface of the AuNPs again. Because the electrostatic repulsion of the negative charge of citrate ions induces the redispersion of AuNPs, a significant color change from blue to purple or even red is observed. The changes in the UV-vis spectra and solution color, induced by ligand exchange reaction, allowed the colorimetric determination of Hg2+ by UV-vis spectroscopy or unaided eye in aqueous solution.
It is well-known that AuNPs display a maximum absorption band at 520 nm. When AuNPs are modified by DETL, the maximum absorption band appears at 650 nm, and absorption band shifts from 520 to 525 nm. The tremendous shift of wavelength may be related to the ligand exchange of thiol group and citrate ions which induces the aggregation of AuNPs. Fig. 1 (A, a) shows the UV-vis spectra of DETL-AuNPs. After the addition of Hg2+, the spectrum displays a red shift, the absorption peak decreases at 650 nm and increase at 525 nm (Fig. 1 (A, b)) because of the redispersion of AuNPs. These results are in agreement with those works [7, 13, 18]. We confirm that color change of DETL-AuNPs solution is induced by the addition of Hg2+. The color changes immediately from blue to red upon the addition of Hg2+ (Fig. 1 (A, b)). TEM images also show that the redispersion of AuNPs occurs after the addition of Hg2+ (Fig. 1 (B)).
Optimization of method
The performance of this Hg2+ method is strongly influenced by the experimental parameters such as reaction time, temperature, and pH in the colloidal solution. Therefore, each detection parameter was optimized in our study, while keeping the other parameters constant. The following parameters were optimized: (a) Sample pH value; (b) Reaction temperature; (c) Reaction time. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) Best sample pH value: 6.6; (b) Optimal temperature: 30 °C; (c) Optimal reaction time: 20 min.
Sensitivity of the assay
Figure 2 shows the colorimetric responses of the DTET-AuNPs upon the addition of various concentrations of Hg2+. As we can see, with the increase of Hg2+ concentrations, an color change from blue to red is displayed in Fig. 2 (B). It is easy to observe the detection effect from the change of color by naked-eye. Besides, the absorbance peaks at 650 nm decrease gradually, for the corresponding increasing at 525 nm. Furthermore, the absorption ratio (A650/A525) linearly grows with the increase of Hg2+ in the range from 0.1 to 0.5 μM and from 0.5 μM to 5 μM, with the correlation coefficients of 0.9845 and 0.9986, respectively. The calibration equation is calculated by fitting the experimental data:
(A) UV-vis absorption spectra of DETL-AuNPs in the presence of different concentrations of Hg2+ (a to k express 0, 0.1, 0.25, 0.35, 0.45, 0.5, 1, 2, 3, 5, 10 μM). (B) Images of DETL-AuNPs containing different concentrations of Hg2+ (0, 0.1, 0.25, 0.35, 0.45, 0.5, 1, 2, 3, 5, 10 μM). (C) Absorption ratio (A650/A525) of DETL-AuNPs versus the concentrations of Hg2+ (0, 0.1, 0.25, 0.35, 0.45, 0.5, 1, 2, 3, 5, 10 μM). Error bars were calculated from three experiments
Where y is the ratio of spectral absorbance A650/A525, and x is the concentration of Hg2+. The detection limit is 0.024 μM (S/N = 3), and the relative standard derivations of triplicate measurements are 1.4%. These results show that this assay has a satisfied sensitivity towards the determination of Hg2+. Table 1 exhibits the different methods for the detection of Hg2+. As shows in Table 1, in comparison with methods in literatures, the linear range of our method is wider than those of other methods including the AuNPs as fluorescent method [24] and 3-mercaptopropionate acid-AuNPs colorimetric method [29]. In addition, the detection limit is lower than that of the SPR [25] and FRET [26] methods.
Selective detection of Hg2+
The above results indicate that the colorimetric method is sensitive to the detection of Hg2+. In this case, the selectivity, the most important factor for a method, needs to be explored. To evaluate the selectivity of the method for Hg2+, various competitors such as Cr3+, Mn2+, Ba2+, Ca2+, Cu2+, Fe2+, Zn2+, Mg2+, Cd2+, and Pb2+ were examined. As shows in Fig. 3, except for Hg2+, none of these metal ions can prevent the DETL-induced aggregation of AuNPs, because the sulfydryl in DETL preferentially bind to Hg2+ compared to other metal ions in chemical affinity. Compared with those works [30, 32,34,34], this method do not need to add other masking agent such as ethylenediamine tetraacetic acid (EDTA), and pyridinedicarboxylic acid (PDCA), which shows effective masking ability toward Ba2+, Pb2+ or Cu2+. The concentration of other ions used in this experiment is 100 times higher than that of Hg2+.
(A) Absorption ratio (A650/A525) of the DETL-AuNPs dispersion in the presence of different metal ions (1 mM). (B) Photographic image of DETL-AuNPs dispersion in the presence of different metal ions (1 mM). Mn+ denotes the mixture of all above ions except Hg2+, and Mn + 1 denotes the mixture of all above ions including Hg2+ (10 μM)
Determination of Hg2+ in real water samples
To evaluate our method for the detection of Hg2+ in real samples, tap water samples from our laboratory, pond water samples from the Lake of Peach (Changsha, China) and river water samples from Hsiang River (Changsha, China) are used for this method. Firstly, we filtered the water samples through 0.22 μm membrane. Then, river water, tap water and pond water were spiked with standard Hg2+ solutions (0.2 μM, 0.5 μM and 4 μM, respectively). The results are summarized in Table. S1 (see in Electronic Supporting Material). As can be observed in Table. S1, the recoveries are within the range from 100.40 to 104.50%. The data are then compared by the AAS measurement. The results are still satisfactory. Therefore, the method has promising feasibility for rapid detection of Hg2+ in real water samples.
Conclusion
We describe a highly selective colorimetric Hg2+ detecting system using DTET-AuNPs in aqueous media. This rapid and reliable detecting system provides additional advantages: (i) high selectivity for Hg2+ over other major interfering metal ions such as Cu2+, Cd2+, and Pb2+ without using any masking agents compared with previous works; (ii) short assay time and lab-friendly condition (aqueous solution, room temperature); (iii) it does not require a fluorescence dye, and simplicity of the use of AuNPs and commercially available DETL as the probe without any modification, design, and complex synthesis; (iv) direct visualization of end result with color change; and (v) low cost due to use of inexpensive instruments. Finally, we hope this nanoprobe can contribute to valuable development of Hg2+ detection strategy.
References
Xue W, Huang D, Zeng G, Wan J, Zhang C, Xu R, Cheng M, Deng R (2018) Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of cd and Pb in river sediments. J Hazard Mater 341:381
Gong X, Huang D, Liu Y, Zeng G, Wang R, Wei J, Huang C, Xu P, Wan J, Zhang C (2018) Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: for heavy metals stabilization and dye adsorption. Bioresour Technol 253:64–71. https://doi.org/10.1016/j.biortech.2018.01.018
Da Q, Gu Y, Peng X, Zhang L, Du S (2018) Colorimetric and visual detection of mercury(II) based on the suppression of the interaction of dithiothreitol with agar-stabilized silver-coated gold nanoparticles. Microchim Acta 185(7):357. https://doi.org/10.1007/s00604-018-2899-y
Butwong N, Kunthadong P, Soisungnoen P, Chotichayapong C, Srijaranai S, Luong JHT (2018) Silver-doped CdS quantum dots incorporated into chitosan-coated cellulose as a colorimetric paper test stripe for mercury. Microchim Acta 185(2):126. https://doi.org/10.1007/s00604-018-2671-3
Huang D, Li Z, Zeng G, Zhou C, Xue W, Gong X, Yan X, Chen S, Wang W, Cheng M (2019) Megamerger in photocatalytic field: 2D g-C3N4 nanosheets serve as support of 0D nanomaterials for improving photocatalytic performance. Appl Catal B Environ 240:153–173. https://doi.org/10.1016/j.apcatb.2018.08.071
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261
Lai C, Liu X, Qin L, Zhang C, Zeng G, Huang D, Cheng M, Xu P, Yi H, Huang D (2017) Chitosan-wrapped gold nanoparticles for hydrogen-bonding recognition and colorimetric determination of the antibiotic kanamycin. Microchim Acta 184(7):2097–2105. https://doi.org/10.1007/s00604-017-2218-z
Wang X, Yang X, Wang N, Lv J, Wang H, Choi MMF, Bian W (2018) Graphitic carbon nitride quantum dots as an “off-on” fluorescent switch for determination of mercury(II) and sulfide. Microchim Acta 185(10):471. https://doi.org/10.1007/s00604-018-2994-0
Liu X, Huang D, Lai C, Zeng G, Qin L, Zhang C, Yi H, Li B, Deng R, Liu S, Zhang Y (2018) Recent advances in sensors for tetracycline antibiotics and their applications. TrAC Trends Anal Chem 109:260–274. https://doi.org/10.1016/j.trac.2018.10.011
Hong M, Zeng B, Li M, Xu X, Chen G (2017) An ultrasensitive conformation-dependent colorimetric probe for the detection of mercury(II) using exonuclease III-assisted target recycling and gold nanoparticles. Microchim Acta 185(1):72. https://doi.org/10.1007/s00604-017-2536-1
Wang C, Tang G, Tan H (2018) Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim Acta 185(10):475. https://doi.org/10.1007/s00604-018-3011-3
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184(1):45–58. https://doi.org/10.1007/s00604-016-1967-4
Qin L, Zeng G, Lai C, Huang D, Zhang C, Xu P, Hu T, Liu X, Cheng M, Liu Y (2017) A visual application of gold nanoparticles: simple, reliable and sensitive detection of kanamycin based on hydrogen-bonding recognition. Sensors Actuators B Chem 243:946–954
Yun W, Jiang J, Cai D, Zhao P, Liao J, Sang G (2016) Ultrasensitive visual detection of DNA with tunable dynamic range by using unmodified gold nanoparticles and target catalyzed hairpin assembly amplification. Biosens Bioelectron 77:421–427
Lai C, Qin L, Zeng G, Liu Y, Huang D, Zhang C, Xu P, Cheng M, Qin X, Wang M (2016) Sensitive and selective detection of mercury ions based on papain and 2,6-pyridinedicarboxylic acid functionalized gold nanoparticles. RSC Adv 6(4):3259–3266. https://doi.org/10.1039/c5ra23157d
Zhou Y, Dong H, Liu L, Li M, Xiao K, Xu M (2014) Selective and sensitive colorimetric sensor of mercury (II) based on gold nanoparticles and 4-mercaptophenylboronic acid. Sensors Actuators B Chem 196:106–111
Feng JJ, Guo H, Li YF, Wang YH, Chen WY, Wang AJ (2013) Single molecular functionalized gold nanoparticles for hydrogen-bonding recognition and colorimetric detection of dopamine with high sensitivity and selectivity. ACS Appl Mater Interfaces 5(4):1226–1231
Duan J, Yin H, Wei R, Wang W (2014) Facile colorimetric detection of Hg2+ based on anti-aggregation of silver nanoparticles. Biosens Bioelectron 57:139–142. https://doi.org/10.1016/j.bios.2014.02.007
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 174:545–553
Kim YR, Mahajan RK, Kim JS, Kim H (2010) Highly sensitive gold nanoparticle-based colorimetric sensing of mercury(II) through simple ligand exchange reaction in aqueous media. ACS Appl Mater Interfaces 2(1):292–295. https://doi.org/10.1021/am9006963
Chen Z, Zhang C, Ma H, Zhou T, Jiang B, Chen M, Chen X (2015) A non-aggregation spectrometric determination for mercury ions based on gold nanoparticles and thiocyanuric acid. Talanta 134:603–606. https://doi.org/10.1016/j.talanta.2014.11.065
Tsai DH, Cho TJ, DelRio FW, Gorham JM, Zheng J, Tan J, Zachariah MR, Hackley VA (2014) Controlled formation and characterization of dithiothreitol-conjugated gold nanoparticle clusters. Langmuir 30(12):3397–3405
Huang CC, Chang HT (2006) Selective gold-nanoparticle-based “turn-on” fluorescent sensors for detection of mercury (II) in aqueous solution. Anal Chem 78(24):8332–8338
Chang HY, Hsiung TM, Huang YF, Huang CC (2011) Using rhodamine 6G-modified gold nanoparticles to detect organic mercury species in highly saline solutions. Environ Sci Technol 45(4):1534–1539. https://doi.org/10.1021/es103369d
Wang L, Li T, Du Y, Chen C, Li B, Zhou M, Dong S (2010) Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury(II) ions. Biosens Bioelectron 25(12):2622–2626. https://doi.org/10.1016/j.bios.2010.04.027
Yu CJ, Tseng WL (2008) Colorimetric detection of mercury (II) in a high-salinity solution using gold nanoparticles capped with 3-mercaptopropionate acid and adenosine monophosphate. Langmuir 24(21):12717–12722
Chen J, Zheng A, Chen A, Gao Y, He C, Kai X, Wu G, Chen Y (2007) A functionalized gold nanoparticles and rhodamine 6G based fluorescent sensor for high sensitive and selective detection of mercury(II) in environmental water samples. Anal Chim Acta 599(1):134–142. https://doi.org/10.1016/j.aca.2007.07.074
Li YL, Leng YM, Zhang YJ, Li TH, Shen ZY, Wu AG (2014) A new simple and reliable Hg2+ detection system based on anti-aggregation of unmodified gold nanoparticles in the presence of O-phenylenediamine. Sensors Actuators B Chem 200:140–146. https://doi.org/10.1016/j.snb.2014.04.039
Huang CC, Chang HT (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Commun (12):1215–1217. https://doi.org/10.1039/b615383f
Ding N, Zhao H, Peng W, He Y, Zhou Y, Yuan L, Zhang Y (2012) A simple colorimetric sensor based on anti-aggregation of gold nanoparticles for Hg2+ detection. Colloids Surf A Physicochem Eng Asp 395:161–167. https://doi.org/10.1016/j.colsurfa.2011.12.024
Gao Y, Li X, Li Y, Li T, Zhao Y, Wu A (2014) A simple visual and highly selective colorimetric detection of Hg2+ based on gold nanoparticles modified by 8-hydroxyquinolines and oxalates. Chem Commun 50(49):6447–6450. https://doi.org/10.1039/c4cc00069b
Si S, Kotal A, Mandal TK (2007) One-dimensional assembly of peptide-functionalized gold nanoparticles: an approach toward mercury ion sensing. J Phys Chem C 111(3):1248–1255
Lou T, Chen Z, Wang Y, Chen L (2011) Blue-to-red colorimetric sensing strategy for hg(2)(+) and ag(+) via redox-regulated surface chemistry of gold nanoparticles. ACS Appl Mater Interfaces 3(5):1568–1573. https://doi.org/10.1021/am200130e
Yu CJ, Cheng TL, Tseng WL (2009) Effects of Mn2+ on oligonucleotide-gold nanoparticle hybrids for colorimetric sensing of Hg2+: improving colorimetric sensitivity and accelerating color change. Biosens Bioelectron 25(1):204–210. https://doi.org/10.1016/j.bios.2009.06.038
Acknowledgements
This study was financially Supported by the Program for the National Natural Science Foundation of China (51879101,51579098, 51779090, 51709101, 51408206, 51521006), the National Program for Support of Top–Notch Young Professionals of China (2014), The Science and Technology Plan Project of Hunan Province (2018SK20410, 2017SK2243, 2016RS3026), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13R17), and the Fundamental Research Funds for the Central Universities (531109200027,531107050978, 531107051080).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 429 kb)
Rights and permissions
About this article
Cite this article
Huang, D., Liu, X., Lai, C. et al. Colorimetric determination of mercury(II) using gold nanoparticles and double ligand exchange. Microchim Acta 186, 31 (2019). https://doi.org/10.1007/s00604-018-3126-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3126-6