Abstract
Cadmium selenide quantum dots were capped with reduced graphene oxide that was modified with thioglycolic acid. The nanocomposite was prepared by 5-min sonication of a solution of graphene oxide, thioglycolic acid, and cadmium(II) nitrate and selenium powder in the presence of NaBH4. X-ray diffraction and transmission electron microscopy were used to characterize the nanocomposite. A glassy carbon electrode (GCE) was modified with this nanocomposite and used for simultaneous determination of dopamine (DA), ascorbic acid (A) and uric acid (UA). The modified GCE was characterized by using cyclic voltammetry and differential pulse voltammetry. Simultaneous determination of AA, DA and UA was accomplished at working voltages of −50, +148 and + 280 mV (all vs. Ag/AgCl), respectively. The voltammetric response to DA is linear in the 4.9 to 74.0 μM concentration range, and the detection limit (defined as 3σ of the blank) is 0.11 μM. The respective data are 0.39–1.0 mM and 66 μM for AA, and 9.0 to 120.0 μM and 0.12 μM for UA. The electrode was successfully applied to the determination of the 3 species in spiked urine samples.

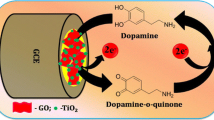
A sonochemical method was applied for synthesizing reduced graphene oxide decorated thioglycolic acid capped cadmium selenide quantum dots. A modified glassy carbon electrode was prepared for simultaneous determination of ascorbic acid, dopamine and uric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semiconductor quantum dots (QDs), which are nanoparticles containing group II – VI or III–V elements, have received more attention because of their various applications such as optoelectronic [1], photochemical [2], catalytic properties [3] and etc. Although, it can be predicted that quantum dots have superior electrochemical properties due to their good biocompatibility, large specific surface area and very small size [4,5,6]. Due to large numbers of papers dealing with QDS, in a critical review M. Amelia et al. discussed [7] the results of electrochemical studies carried out on CdSe and CdTe nanocrystals.
Despite good electronic properties of QDs, they play an exiguity role in electrochemical applications because of their low electrochemical conductivity. In order to improve the QDs conductivity, conjugation with gold nanoparticles and carbon based materials such as carbon nanotubes (CNT) [8] and graphene [9, 10] has drawn considerable attention. Several methods have been used to synthesize decorated graphene with QDs [11,12,13], but most of them are expensive, complex and time-consuming. Thus, it is important to find the facile, fast, efficient and economical method to fabricate the graphene-QD nanocomposites to enhance electrochemical performance. The sonochemical method and high intensity ultrasound can be used for the production of novel materials and provides an unusual route to known materials or modification of nanomaterials [14,15,16].
Ascorbic acid (AA), dopamine (DA) and uric acid (UA) always coexist in the extra cellular fluids of the central nervous system and serum in mammals, and they are crucial molecules for physiological reactions in human metabolism [17]. However, selective detection of DA, AA, and UA, and even their simultaneous detection have received tremendous attention in biomedical chemistry, as well as diagnostic and pathological research owing to their similar properties [18]. Thus, numerous methods including chromatography [19], fluorimetry [20], chemiluminescence [21], voltammetry [22], ultraviolet-visible spectrophotometry [23], capillary electrophoresis [24] and etc. have been reported for the determination of these species in the literature. Among these methods, the electrochemical methods have many advantages for determination of these species, such as direct detection, high sensitivity, cost effectiveness, and fast response measurement. However, simultaneous determination of these species is difficult because overlapping of the oxidation peaks of these three species is the main disadvantage of the electrochemical technique. So, simultaneous determination of DA, UA, and AA has been a basic goal in many studies in electrochemical field.
In this study, a sonochemical method was applied for synthesized reduced graphene oxide which decorated thioglycolic acid capped cadmium selenide quantum dots (RGO-CdSe QDs) and a modified glassy carbon electrode based on RGO-CdSe QDs was prepared for simultaneous determination of, dopamine, ascorbic acid and uric acid. The parameters were optimized and a convenient, simple, rapid, and direct detection method at low concentration was developed for the determination of AA, DA, and UA. This system showed good performances such as high sensitivity, good selectivity and reproducibility, short response time, and wide linear range. Finally, the AA, DA, UA content in urine samples was determined with satisfactory results.
Experimental
Materials and apparatus
For this experiment, graphite powder was purchased from Fluka (www.sigma-aldrich.com). Ascorbic avid (AA), dopamine (DA), usic acid (UA), Cd(NO3)2.4H2O and selenium powder (99.9%), Na2HPO4, NaH2PO4, H3PO4 and NaOH were purchased from Merck (www.merck.com). Phosphate buffer (0.1 M) at different pH was prepared by mixing stock solutions of Na2HPO4 and NaH2PO4, and the pH was adjusted with H3PO4 and NaOH. All other chemicals were of analytical grade and used without further purification. In all experiments doubly distilled water was also used throughout the experiments. Artificial human urine samples were provided as a real sample.
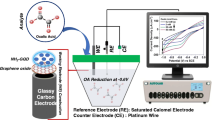
All electrochemical measurements were carried out using a potentiostat/galvanostat PGSTAT302 (Metrohm Autolab B.V., Utrecht, and the Netherlands). A three electrode cell containing RGO-CdSe QD/GCE, platinum wire and Ag/AgCl electrode was used as working electrode, the counter electrode and the reference electrode respectively. The structure and morphology of samples were characterized by X-ray diffraction (XRD, BRUKER, D8 ADVANCE, Cu Kα radiation), and transmission electron microscopy (TEM, Zeiss - EM10C - 80 KV), respectively. Also, pH measurements were made with a pH meter (DENVER 780) equipped with a Metrohm glass electrode.
Synthesis of RGO-CdSe QDs/GCE
In the first step graphene oxide (GO) was synthesized from graphite powder by a well-known Hummers method [25]. The synthesized GO was dispersed in water by the ultrasonic to form homogeneous suspension for further use. In the second step the reduced graphene oxide cadmium selenide (RGO-CdSe QDs) was synthesized by a fast sonication method. In the typical synthesis procedure, 0.4 mmol Cd(NO3)2.4H2O and 0.75 mmol TGA were added to 75.0 mL GO suspension and the pH was adjusted to 11.0 using 1.0 M NaOH. Then this solution was added to 25.0 mL solution that contains 0.15 mmol Se powder and 0.45 mmol NaBH4. The resulting solution, sonicated (by probe sonication) for 5.0 min (under argon gas). In this step graphene oxide was reduced to RGO and in the presence of CdSe QDs, RGO-CdSe QDs as a final product was produced. Finally the product was separated with centrifuge, washed with water three times and dried in vacuum oven.
Modified electrode preparation
Prior to coating, a glassy carbon electrode (GCE) (3.0 mm inner diameter) was successively polished with alumina powder and rinsed thoroughly with 1:1 deionized water, ethanol and deionized water each for 90 s, and dried at room temperature. For electrode modification, first 1.0 mg of RGO-CdSe QDs was dispersed in 1.0 mL deionized water and sonicated for 3 min. Then 2.0 μL of prepared solution was applied to the clean GCE and allowed to dry in air at room temperature. This electrode denoted as RGO-CdSe QD/GCE. For comparative studies, RGO/ GCE was prepared in the same way.
General procedure
Stock solution of DA, AA, and UA was freshly prepared prior to use. Electrochemical experiments were carried out in the phosphate buffered (pH = 7.0) of DA, AA, and UA. Primarily cyclic voltammetry was applied from −0.3 to 0.5 V. For further investigation of electrocatalytic and determination properties, the RGO-CdSe QD/GCE was chosen, also, differential pulse voltammetric method (DPV) was used to obtain better sensitivity and resolution because enhanced analytical signals can be achieved by eliminating the non-Faradaic currents that occur with CV. Finally, the differential pulse voltammograms were recorded from −0.2 to 0.4 V (DPV were performed with pulse potential of 50.0 mV, pulse duration of 50.0 ms and pulse period of 0.2 s). All measurements were carried out at room temperature (25 ± 1 °C).
Results and discussion
Structural and morphological characterization
First the synthesized materials were characterized with X-ray diffraction as shown in Fig. 1 a-d. Figure 1a shows a typical XRD pattern of the GO sample with a single peak at 12.6°, which is assigned to the (0 0 1) lattice plane of GO. Compared to the typical diffraction peak at 12.6°, those of RGO (Fig.1b) and RGO-CdSe QDs (Fig. 1d) have smaller intensities that shows reduction of GO and decoration of graphene sheet with CdSe nanoparticles [26]. Also the diffraction pattern of CdSe QDs (Fig. 1.c), is in good agreement with the standard patterns for CdSe (JCPDS card no. 77–0046) [26]. In the XRD pattern of RGO-CdSe QDs along with the peaks of CdSe QDs, an additional peak was noticed at a diffraction angle of 12.6° that indicates the existence of little non reduced graphene oxide (GO) in this composite.
In addition, the XRD pattern of the RGO-CdSe QDs and CdSe QDs exhibited broader and weaker diffraction peaks, reveling weaker crystallinity and nanosize of these materials. The morphology of the synthesized materials was characterized by transition electron microscopy (TEM) and the results are shown in Fig. 2. Figure 2a shows the smooth surface of the graphene sheet before the growth of CdSe QDs and Fig. 2b shows the well dispersed of CdSe QDs nanoparticles with spherical shape on the surface of graphene sheet. Sizes of these nanoparticles are varied between 5 and 20 nm. The TEM images again confirm the successful decoration of CdSe QDs on the graphene sheets.
Electrochemical characterization
Cyclic voltammetric detection of AA, DA, and UA
The electrocatalytic behavior of the bare and modified electrodes for the oxidation of AA, DA and UA was studied by cyclic voltammetry. Fig. 3 (a-c) shows the cyclic voltammograms of a bare GCE, RGO and RGO-CdSe QD modified electrodes in a solution containing of 0.1 mM of each AA (A), DA (B) and UA (C) in 0.1 M phosphate buffer (pH 7.0). The electrochemical behavior of AA, DA and UA at bare, RGO, and RGO-CdSe QD modified electrodes are approximately similar. In the case of AA (Fig. 3a), the oxidation peak corresponds to the oxidation of hydroxyl groups to carbonyl groups in furan ring of AA and for DA (Fig. 3b), two approximately reversible peaks were observed to corresponds to two-electron oxidation of DA to dopamine quinone and the subsequent reduction of dopamine quinone to DA. For UA (Fig. 3c), a well-defined oxidation peak appears and a broad reduction peak that reveals the UA is first oxidized to quinonoid, and then undergoes a rapid chemical reaction. In continues and shown in Fig. 3 (a-c) DA and AA, the peak currents on the modified electrode remarkably increased and for UA, along with the increasing of peak currents the baseline current decreased on the modified electrode that improved signal to noise ratio. Also, the oxidation peak potential on the modified electrode negatively shifted compared to other electrodes for DA (ΔEp decreased to 70 mV), UA and especially for AA. These results were achieved due to the synergistic effect between RGO and CdSe QD nanoparticles and again confirm good coupling between catalytic activity of CdSe QD nanoparticles and conductivity of RGO for oxidation and reduction of DA, UA, and AA. In addition, existence of some hydroxyl groups causes improving electrocatalytic activity for oxidation AA, DA, UA [27].
Optimization of method
The following parameters were optimized: (a) Sample pH value; (b) scan rate and the respective data and Figures S1 and S2 are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) Best sample pH value: 7.0 (b) Optimal scan rate: 50 mV s−1.
Simultaneous determination of AA, DA, and UA
For simultaneous determination of DA, AA, and UA, differential pulse voltammetry (DPV) was carried out in the potential range of −200 to 400 mV for a solution containing a mixture of DA, AA and UA. As is well seen (Fig. 4), three well-defined and resolved peaks at about −50, 130, and 280 mV (vs. Ag/AgCl) were observed, corresponding to the differential pulse voltammograms of AA, DA, and UA, respectively. Also, Peak separations between DA and AA, DA and UA, and UA and AA were 135, 150, and 285 mV, respectively, suggesting that the RGO-CdSe QD/GCE was appropriate for simultaneous determination of three species as an electrochemical sensor.
In addition, peak potentials of these three analyses were kept almost unchanged and the peak currents of the detected substance increased linearly with the increase of its concentrations in wide range. The electro-oxidation processes of AA, DA and UA in the mixture when the concentration of one species changed other two species are kept constant was also investigated and the results are shown in Fig. 5 The voltammetric response to DA is linear in the 4.9 to 74 μM concentration range with electrochemical sensitivity of 0.16 μAμM−1 cm−2, and the detection limit (defined as 3σ of the blank) is 0.11 μM. The respective data are 0.39–1.0 mM, 9.0 × 10−3 μAμM−1 cm−2 and 66 μM for AA, and from 9.0 to 120 μM, 0.06 μAμM−1 cm−2 and 0.12 μM for UA. These results demonstrate that individual or simultaneous determination of AA, DA and UA on RGO-CdSe QD/GCE can be achieved with good sensitivity and selectivity. These good analytical parameters (including detection limit and linear range and sensitivity) are due to the excellent electrocatalytic of graphene and CdSe nanoparticles and synergistic effect between two components towards these analytes. A comparison between this work and some previously reported procedures for determination of AA, DA, and UA is given in Table 1. As displayed in this table, the analytical parameters including detection limit and linear range using RGO-CdSe QD/GCE are better or comparable to the results reported for simultaneous determination of these analytes at different modified electrode surfaces.
Differential pulse voltammetry of RGO-CdSe QD/GCE in 0.1 M phosphate buffer (pH 7.0)(a) Containing 14.9 μM DA, 14.9 μM UA and different concentrations of AA from 3.9 × 10−4 to 1.0 × 10−3 M at working voltages of −50 mV (vs. Ag/AgCl) (b) Containing 1.0 mM AA, 14.8 μM UA and different concentrations of DA from 4.9 × 10−6 to 7.4 × 10−5 M at working voltages of +148 mV (vs. Ag/AgCl) (c) Containing 1.0 mM AA, 4.9 μM DA and different concentrations of UA from 9.0 × 10−6 to 1.2 × 10−4 M at working voltages of +280 mV (vs. Ag/AgCl) Pulse time: 50 ms, potential step: 5 mV, Sweep rate: 10 mV s−1
The reversibility of the RGO-CdSe QD/GCE was investigated by using DPV for 10 repetitive measurements containing these three analyses. The relative standard deviations (RSD) for AA, DA, and UA were 2.74, 2.45, and 2.66%, respectively that confirm the modified electrode for determination of these analyses was stable.
Real sample analysis
The utilization of the modified GCE in real samples was studied by the standard addition method in artificial human urine samples [36]. All samples were diluted with phosphate buffer (pH 7.0) without any pretreatment and then appropriate amounts of these diluted samples were transferred to the electrochemical cell for the determination of each species using DPV. The results are presented in Table 2. Results show that this electrode was reliable enough for practical determination of AA, DA, and UA.
Conclusion
In this work, RGO-CdSe QDs nanocomposite was synthesized with a fast sonication method and was used to modify glassy carbon electrodes (RGO-CdSe QDs/GCE) for simultaneous electrochemical determination of AA, DA and UA. Results showed that RGO-CdSe QDs/GCE exhibited better catalytic activity compare to GCE and RGO/GCE toward electrooxidation of DA, AA and UA and showed this electrode can be used for individual or simultaneous determination of these analyses with good sensitivity and selectivity. Also this electrode showed good analytical parameters (including detection limit and linear range) that can be due to the synergistic effect between graphene and CdSe nanoparticles in the RGO-CdSe QDs nanocomposite towards and existing of some hydroxyl group (due to little of non-reduce graphene oxide) in this nanocomposite. In addition, this electrode was successfully applied in human urine samples for confirming practical application of this electrode for determination of AA, DA and UA.
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Chen D, Tang L, Li J (2010) Graphene-based materials in electrochemistry. Chem Soc Rev 39:3157–3180
Kim KS, Kim IJ, Park SJ (2010) Influence of ag doped graphene on electrochemical behaviors and specific capacitance of polypyrrole-based nanocomposites. Synth Met 160:2355–2360
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937
Peng X, Manna L, Yang W, Wickham J, Scher E, Kadavanich A, Alivisatos AP (2000) Shape control of CdSe nanocrystals. Nature 404:59–61
Amelia M, Lincheneau C, Silvi S, Credi A (2012) Electrochemical properties of CdSe and CdTe quantum dots. Chem Soc Rev 41:5728–5743
Olek M, Büsgen T, Hilgendorff M, Giersig M (2006) Quantum dot modified multiwall carbon nanotubes. J Phys Chem B 110:12901–12904
Cao A, Liu Z, Chu S, Wu M, Ye Z, Cai Z, Chang Y, Wang S, Gong Q, Liu Y (2010) A facile one-step method to produce graphene–cds quantum dot nanocomposites as promising optoelectronic materials. Adv Mater 22:103–106
Kim YT, Han JH, Hong BH, Kwon YU (2010) Electrochemical synthesis of cdse quantum-dot arrays on a graphene basal plane using mesoporous silica thin-film templates. Adv Mater 22:515–518
Oh W-C, Chen M, Cho K, Kim C, Meng Z, Zhu L (2001) Synthesis of graphene-CdSe composite by a simple hydrothermal method and its photocatalytic degradation of organic dyes. Chin J Catal 32:1577–1583
Gao Z, Liu N, Wu D, Tao W, Xu F, Jiang K (2012) Graphene–CdS composite, synthesis and enhanced photocatalytic activity. Appl Surf Sci 258(s):2473–2478
Lei Y, Li R, Chen F, Xu J (2014) Hydrothermal synthesis of graphene–CdS composites with improved photoelectric characteristics. J Mater Sci Mater Electron 25:3057–3061
Zhu J, Liu S, Palchik O, Koltypin Y, Gedanken A (2000) A novel Sonochemical method for the preparation of Nanophasic sulfides: synthesis of HgS and PbS nanoparticles. J Solid State Chem 153:342–348
Bang BJH, Suslick KS (2010) Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater 22:1039–1059
Xu H, Zeiger BW, Suslick KS (2013) Sonochemical synthesis of nanomaterials. Chem Soc Rev 42:2555–2567
Manjunatha H, Nagaraju DH, Suresh GS, Venkatesha TV (2009) Detection of uric acid in the presence of dopamine and high concentration of ascorbic acid using pdda modified graphite electrode. Electroanalysis 21:2198–2206
Chih YK, Yang MC (2013) An 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)-immobilized electrode for the simultaneous detection of dopamine and uric acid in the presence of ascorbic acid. Bioelectrochemistry 91:44–51
Guan C, Ouyang J, Li Q, Liu B, Baeyens W (2000) Simultaneous determination of catecholamines by ion chromatography with direct conductivity detection. Talanta 50:1197–1203
Nohta H, Yukizawa T, Ohkura Y, Yoshimura M, Ishida J, Yamaguchi M (1997) Aromatic glycinonitriles and methylamines as pre-column fluorescence derivatization reagents for catecholamines. Anal Chim Acta 344:233–240
Li J, Lue J (1997) Flow-injection/chemiluminescence assays of catecholamines, Chinese J of. Anal Chem 25:314–317
Downard AJ, Roddick AD, Bond AM (1995) Covalent modification of carbon electrodes for voltammetric differentiation of dopamine and ascorbic acid. Anal Chim Acta 317:303–310
El-Zohry AM, Hashem EY (2013) Environmental method to determine dopamine and ascorbic acid simultaneously via derivative spectrophotometry. J Spectrosc, Article ID 260376, 1–7
Zhao S, Wang J, Ye F, Liu YM (2008) Determination of uric acid in human urine and serum by capillary electrophoresis with chemiluminescence detection. Anal Biochem 378:127–131
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Wang Y, Yao HB, Wang XH, Yu SH (2013) One-pot facile decoration of CdSe quantum dots on graphene nanosheets: novel graphene-CdSe nanocomposites with tunable fluorescent properties. Nanoscale 5:827–844
Safavi A, Maleki N, Moradlou O, Tajabadi F (2006) Simultaneous determination of dopamine, ascorbic acid, and uric acid using carbon ionic liquid electrode. Anal Biochem 359:224–229
Deng K, Li X, Huang H (2016) A glassy carbon electrode modified with a nickel(II) norcorrole complex and carbon nanotubes for simultaneous or individual determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:2139–2145
Wu F, Huang T, Hu Y, Yang X, Ouyang Y, Xie Q (2016) Differential pulse voltammetric simultaneous determination of ascorbic acid, dopamine and uric acid on a glassy carbon electrode modified with electroreduced graphene oxideand imidazolium groups. Microchim Acta 183:2539–2546
Wang X, Wu M, Tang W, Zhu Y, Wang L, Wang Q, He P, Fang Y (2013) Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid using a palladium nanoparticle/graphene/chitosan modified electrode. J Electroanal Chem 695:10–16
Yang L, Liu D, Huang J, You T (2014) Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sensors Actuators B Chem 193:166–172
Yan S, Li X, Xiong Y, Wang M, Yang L, Liu X, Li X, Alshahrani LAM, Liu P, Zhang C (2016) Simultaneous determination of ascorbic acid, dopamine and Uri acid using a glassy carbon electrode modified with the nickel(II) -bis(1,10-phenanthroline) complex and single-walled carbon nanotubes. Microchim Acta 183:1401–1408
Zhao D, Fan D, Wan J, Xu C (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 182:1345–1352
Xing L, Ma Z (2016) A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:257–263
Tsierkezos NG, Ritter U, Thaha YN, Downing C, Szroeder P, Scharff P (2016) Multi-walled carbon nanotubes doped with boron as an electrode material for electrochemical studies on dopamine, uric acid, and ascorbic acid. Microchim Acta 183:35–47
Chutipongtanate S, Thongboonkerd V (2010) Systematic comparisons of artificial urine formulas for in vitro cellular study. Anal Biochem 402:110–112
Acknowledgements
We gratefully acknowledge the support of this work by the Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 330 kb)
Rights and permissions
About this article
Cite this article
Tavakolian, E., Tashkhourian, J. Sonication-assisted preparation of a nanocomposite consisting of reduced graphene oxide and CdSe quantum dots, and its application to simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim Acta 185, 456 (2018). https://doi.org/10.1007/s00604-018-2988-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2988-y