Abstract
A fluorometric assay is described for sulfide ions determination. It is based on the finding that the oxidation of the non-fluorescent substrate thiamine (TH) by Cu(II) in basic solution to form fluorescent thiochrome is inhibited by sulfide ions. This results in a decrease in fluorescence intensity which is proportional to the concentration of sulfide ions. Under the optimized conditions, the decrease in fluorescence, best measured at excitation/emission wavelengths of 370/440 nm, decreases linearly in the 0.03 to 2.5 μM sulfide ions concentration range. The detection limit is 20 nM. The method shows excellent selectivity over many potentially interfering ions and has been successfully applied to the determination of sulfide ions in spiked tap water, lake water and the synthetic wastewater samples. The method is time-saving and environmentally friendly, and in our perception shows a great potential in water quality inspection and environmental monitoring.

A fluorescent assay for sulfide ions detection is proposed based on its inhibitory effect on the oxidation of thiamine by Cu(II) ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfide ions (S2−), a pollutant index for water, are widely distributed in natural and waste water samples [1, 2]. They have been frequently used in many industrial locations, such as tanneries, petroleum refineries, printing, textile, dyes, cosmetic or paper manufacturing plants, where they are either employed as reactant or produced as a by-product of manufacturing or industrial processes [3]. In acidic conditions, S2− are protonated and converted to HS− or H2S [4], becoming more hazardous, which will cause serious environmental problems and present a danger to human health. Therefore, developing highly sensitive methods for S2− detection is of great importance. To date, various kinds of analytical techniques have been utilized for S2− detection, such as titration [5], high performance liquid chromatography [6], capillary electrophoresis [7], electrochemisty [8] and chemiluminescence [9] and fluorimetry [10, 11]. Among these techniques, fluorimetry has attracted significant attention owning to its short detection time, easy operability and high sensitivity [12,13,14].
Actually, many fluorescent probes have been designed for S2− detection based on their reduction ability, nucleouphilicity or binding affinity to metal ions [15,16,17,18,19,20]. However, it is still challenging for S2− detection. For examples, most of the fluorescent probes are toxic, poorly water-soluble and not utilized in totally aqueous media or they require long response time. Thus, environment-friendly and water-soluble noble metal clusters and carbon quantum dots have been utilized for S2− detection [21, 22]. While the high cost of noble metal salts along with long hydrolysis time limit their further applications. Therefore, establishing time-saving and cost-effective methods is urgently demanded and challenging.
Thiamine (TH), known as vitamin B1, which shows the advantages of low cost, water-solubility and easy accessibility, have been used as non-fluorescent substrate [23]. It can be oxidized by Cu2+ to obtain fluorescent thiochrome under basic conditions [24]. However, the applications of Cu2+-TH system for fluorescent detection have been rarely studied.
In this study, a novel method for S2− detection is proposed base on Cu2+-TH system, where the high binding affinity between Cu2+ and S2− suppress the oxidation of TH resulting in the decrease of fluorescence. Based on the above facts, we develop a novel, sensitive and selective method for S2− detection. This method is time-saving without the utilization of fluorescent nanomaterials or probes, which shows high potential in determination of S2− in real samples.
Experimental section
Materials
Na2S•9H2O, NaH2PO4, Na2HPO4, Na4P2O7•10H2O and thiamine were supplied by Aladdin Reagent Company (Shanghai, China, www.aladdin-e.com). KNO3, NaNO3, Ca(NO3)2•4H2O, Zn(NO3)2•6H2O, Fe(NO3)3•9H2O, Cu(NO3)2•3H2O, NaOH and NaI were obtained from Sigma Aldrich (St. Louis, USA, www.sigmaaldrich.com). NaF, NaCl, NaBr, Na2CO3, NaHCO3, Na2SO4, Na2SO3, Na2S2O3•5H2O, NaAc and NaNO3 were purchased from Damao Chemical Reagent Factory (Tianjin, China, www.dmreagent.com). All reagents were analytical grade and used as received.
Apparatus
Fluorescence measurements were performed on a RF-6000 spectrofluorometer (Shimadzu, Japan, www.shimadzu.com). Both the excitation and emission slits were set as 5.0 nm.
Procedure for S2− detection
The fluorescent analysis for S2− detection was realized as follows. 10 μL of 0.4 mM Cu(NO3)2, 10 μL of various concentrations of freshly prepared of Na2S, 880 μL of 0.05 M NaOH and 100 μL of 1.0 mM TH were added sequentially into a 1.5 mL tube, mixed thoroughly and incubated at 20 °C for 15 min. Then, the mixture was transferred for fluorescent measurements. The fluorescence spectra of the mixture were recorded from 390 nm to 600 nm excited at 370 nm. The same detection procedures were used for the selectivity study, except for that S2− was replaced by other ions or the S2− was added simultaneously with other ions.
Detection in water samples
Tap water and lake water samples were collected from our lab and the Jiazi Lake of University of Jinan, respectively. These samples were filtered through a 0.22 μm membrane and diluted to 10 times and spiked with known concentrations of S2−. The synthetic wastewater samples were prepared according to previous study [25]. In detail, phenol, NaCl, CaCl2, CH3COONa, Na2CO3, KSCN (each of them is 25 mg) and 7.5 mg of (NH4)2SO4 were put into a beaker. Then 50 mL of distilled water were added. Finally, S2− with the finally concentration of 0.1 mM and 0.2 mM were introduced. The same procedures as the standard method was used for S2- detection in spiked water and the synthetic wastewater samples.
Results and discussion
Mechanism of S2− detection
Based on the facts that TH can be oxidized by Cu2+ and the solubility product constant of CuS is 6.3 × 10−36, a possible mechanism of the Cu2+-TH-based system for S2− detection is proposed. Firstly, a remarkable fluorescence signal is found when Cu2+ and TH are simultaneously added (a, Fig. 1), due to the oxidation of TH by Cu2+to fluorescent thiochrome [24]. Upon addition of S2− to the Cu2+-TH system, the oxidation process of TH is inhibited with a dramatic decrease of the fluorescence (b, Fig. 1), which may be caused by the binding of Cu2+ to S2− due to their extremely low solubility product constant. In sharp contrast, barely any fluorescence signal is observed when Cu2+ (c, Fig. 1) or TH (d, Fig. 1) is individually introduced to NaOH solutions, suggesting that both Cu2+ and TH show no fluorescence. Based on the above principles, a novel fluorescent method for S2− detection is developed (Scheme 1).
Optimization of detection conditions
To achieve the best performance for S2− detection, several parameters including the concentrations of NaOH, Cu2+ and thiamine, incubation temperature and time are studied. We use the decrease of fluorescence intensity, that is, F0-F (ΔF, where F0 and F are the fluorescence intensity of the Cu2+-TH system in the absence and presence of S2−, respectively), to optimize the detection conditions. The optimum conditions should be as follows: (a) the concentration of NaOH is 44.0 mM (Fig. S1), (b) the concentrations of Cu2+ and TH are 4.0 μM (Fig. S2) and 0.1 mM (Fig. S3), respectively, (c) the incubation temperature is 20 °C (Fig. S4) and the time is 15 min (Fig. S5).
Analytical performance of the assay
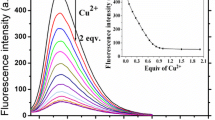
Under the optimized conditions, the analytical performance of this method for S2− detection is evaluated by introduction of various amounts of S2− into the Cu2+-TH system. With the increasing of S2− concentration, the fluorescence intensity at 440 nm decrease gradually (Fig. 2a), while the ΔF increases systematically and reaches a plateau when the S2− concentration is up to 6.0 μM (Fig. 2b). The ΔF displays a good linear relationship versus S2− concentration ranging from 0.03 μM to 2.5 μM. The regression equation is ΔF = 5521.34c (μM) +175.88 (R2 = 0.997). The detection limitation is down to 0.02 μM at a signal-to-noise of 3, which is far below the maximum level of S2− (15.0 μM) in drinking water estimated by the World Health Organization. Compared with previously published methods for S2− detection, the proposed method shows comparable or even better sensitivity and detection linear range (Table S1). The repeatability of this method is demonstrated by six repeated measurements of 2.0 μM of S2− and the relative standard deviation (RSD) of 0.85% is obtained. This result indicates this method is reliable. Moreover, the Cu2+-TH-based method is simple without the need of preparation fluorescent nanomaterials or probes and environment-friendly by using water-soluble TH as non-fluorescent substrate instead of organic and toxic fluorescent probes.
a Fluorescence emission spectra of Cu2+-TH system upon the addition of various concentrations of S2−. From top to down, the concentration of S2− are 0, 0.03, 0.1, 0.3, 0.5, 0.7, 1.0, 1.3, 1.5, 1.7, 2.0, 2.5, 3.0 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 μM. b The ΔF at 440 nm as a function of S2− concentration. Inset shows the linear plot between ΔF and S2− concentration
Selectivity
In order to verify the specificity of this method for S2− detection, the fluorescence responses of Cu2+-TH system towards other anions including F−, Cl−, Br−, I−, SO42−, SO32−, S2O32−, H2PO4−, HPO42−, P2O74−, AcO−, NO3−, CO32−, HCO3−, K+, Na+, Ca2+, Zn2+ and Fe3+ both in the absence and presence of S2− are tested and the results are shown in Fig. 3. No obvious changes of the fluorescence intensity are found when these ions are individually added into the system without the introduction of S2−, while the fluorescence intensity decreases dramatically after the addition of S2−. In addition, the effects of these ions on the fluorescence responses are negligible when they are in coexistence with S2−. The above results demonstrate the high selectivity of this method for S2− detection. However, if the concentrations of these ions exceed their tolerable concentrations, it may cause an effect on S2− detection.
Fluorescence responses of Cu2+-TH system after the addition of S2− and other ions separately or introduction of S2− and other ions at the same time. From left to right, they are F−, Cl−, Br−, I−, SO42−, SO32−, S2O32−, H2PO4−, HPO42−, P2O74−, AcO−, NO3−, CO32−, HCO3−, K+, Na+, Ca2+, Zn2+, Fe3+ and the blank. For the absence of S2−, the concentrations of F−, Cl−, Br−, SO42−, AcO− NO3− and CO32− are 2.0 mM, I−, SO32−, S2O32−, H2PO4−, HPO42−, P2O74−, HCO3−, K+, Na+ and Ca2+ are 0.2 mM, Zn2+ is 0.02 mM, Fe3+ and S2− are 2.0 μM. For the presence of S2−, The concentrations of F− and Cl− are 2.0 mM, Br−, SO42−, AcO−, CO32−, HCO3−, K+, Na+ and Ca2+ are 0.2 mM, I−, HPO42−, P2O74−, H2PO4−, NO3− and Zn2+ are 0.02 mM, SO32−, S2O32−, Fe3+, and S2− are 2.0 μM. Error bar demonstrates the standard deviations of three independent measurements
Analysis of S2− in real samples
To demonstrate the potential application of this method in real samples, the proposed method is used to determine S2− in tap water, lake water and the synthetic wastewater samples. S2− are not detected in either tap water or lake water sample. Then the samples spiked with known concentrations of S2− are analyzed and the results are shown in Table 1. Moreover, this method is used for the analysis of S2− in synthetic wastewater samples and the results are shown in Table S2. Satisfactory recoveries of S2− detection in water samples and acceptable RSD indicate that the Cu2+-TH-based method is highly feasible for S2− determination in real samples.
Conclusions
In summary, a simple, cost effective and environment-friendly method for S2− detection is developed based on Cu2+-TH system. The strong interaction between Cu2+ and S2− through CuS formation inhibits the oxidation reaction of TH by Cu2+ resulting in fluorescence decrease, which can be used for highly sensitive and selective for S2− detection. Moreover, this method has been successfully applied to detect S2− in real samples with satisfactory results. To the best of our knowledge, this is the first time that Cu2+-TH system has been employed for S2− detection and it may pave the way for the wide applications of Cu2+-TH system in trace analysis.
References
Ni PJ, Sun YJ, Dai HC, Hu JT, Jiang S, Wang YL, Li Z, Li Z (2015) Colorimetric detection of sulfide ions in water samples based on the in situ formation of Ag2S nanoparticles. Sensors Actuators B Chem 220:210–215
Liao H, Hu LZ, Zhang YZ, Yu XR, Liu YL, Li R (2018) A highly selective colorimetric sulfide assay based on the inhibition of the peroxidase-like activity of copper nanoclusters. Microchim Acta 185:143
Shanmugaraj K, Ilanchelian M (2016) Colorimetric determination of sulfide using chitosan-capped silver nanoparticles. Microchim Acta 183:1721–1728
Weng H, Yan B (2017) A Eu(III) doped metal-organic framework conjugated with fluorescein-labeled single-stranded DNA for detection of cu(II) and sulfide. Anal Chim Acta 988:89–95
Balasubramanian S, Pugalenthi V (2000) A comparative study of the determination of sulphide in tannery waste water by ion selective electrode (ISE) and iodimetry. Water Res 34:4201–4206
Rembisz Z, Bzdurska D, Obiedzinska J, Martinez-Manez R, Zakrzewski R (2015) A derivatization approach using pyrylium salts for the sensitive and simple determination of sulfide in spring water by high performance liquid chromatography. J Chromatogr A 1407:184–192
Daunoravicius Z, Padarauskas A (2002) Capillary electrophoretic determination of thiosulfate, sulfide and sulfite using in-capillary derivatization with iodine. Electrophoresis 23:2439–2444
Su YZ, Yang SY, Liu WP, Qiao L, Yan J, Liu YJ, Zhang SS, Fang YP (2017) Visible light photoelectrochemical sulfide sensor based the use of TiO2 nanotube arrays loaded with Cu2O. Microchim Acta 184:4065–4072
Liu BB, Han SQ (2016) Determination of trace hydrogen sulfide by using the permanganate induced chemiluminescence of carbon dots. Microchim Acta 183:3087–3092
Jiang HE, Liu Y, Luo WF, Wang YJ, Tang XL, Dou W, Cui YM, Liu WS (2018) A resumable two-photon fluorescent probe for Cu2+ and S2− based on magnetic silica core-shell Fe3O4@SiO2 nanoparticles and its application in bioimaging. Anal Chim Acta 104:91–99
Na WD, Qu ZY, Chen XQ, Su XG (2018) A turn-on fluorescent probe for sensitive detection of sulfide anions and ascorbic acid by using sulfanilic acid and glutathione functionalized graphene quantum dots. Sensors Actuators B Chem 256:48–54
Zhang N, Si YM, Sun ZZ, Chen LJ, Li R, Qiao YC, Wang H (2014) Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal Chem 86:11714–11721
Fan C, Lv XX, Liu FJ, Feng LP, Liu M, Cai YY, Liu H, Wang JY, Yang YL, Wang H (2018) Silver nanoclusters encapsulated into metal-organic frameworks with enhanced fluorescence and specific ion accumulation toward the microdot array-based fluorimetric analysis of copper in blood. ACS Sens 3:441–450
Feng LP, Sun ZZ, Liu H, Liu M, Jiang Y, Fan C, Cai YY, Zhang S, Xu JH, Wang H (2017) Silver nanoclusters with enhanced fluorescence and specific ion recognition capability triggered by alcohol solvents: a highly selective fluorimetric strategy for detecting iodide ions in urine. Chem Commun 53:3466–3469
Lippert AR, New EJ, Chang CJ (2011) Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 133:10078–10080
Xuan WM, Sheng CQ, Cao YT, He WH, Wang W (2012) Fluorescent probes for the detection of hydrogen sulfide in biological systems. Angew Chem Int Ed 51:2282–2284
Liu CR, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M (2011) Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed 50:10327–10329
Xu Z, Xu L, Zhou J, Xu YF, Zhu WP, Qian XH (2012) A highly selective fluorescent probe for fast detection of hydrogen sulfide in aqueous solution and living cells. Chem Commun 48:10871–10873
Li H (2018) A regenerated "turn on" fluorescent probe for sulfide detection in live cells and read samples based on dihydroxyhemicyanine-Cu2+ dye. Anal Chim Acta 1010:69–75
Zheng FY, Wen M, Zeng F, Wu SZ (2013) A robust, water-soluble and low cytotoxic fluorescent probe for sulfide anion achieved through incorporation of betaine. Sensors Actuators B Chem 188:1012–1018
Bai XL, Xu SY, Wang LY (2018) Full-range pH stable Au-clusters in nanogel for confinement-enhanced emission and improved sulfide sensing in living cells. Anal Chem 90:3270–3275
Yu NX, Peng HL, Xiong H, Wu XQ, Wang XY, Li YB, Chen LX (2015) Graphene quantum dots combined with copper(II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim Acta 182:2139–2146
Tan HL, Li Q, Zhou ZC, Ma CJ, Song YH, Xu FG, Wang L (2015) A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal Chim Acta 856:90–95
Perez-Ruiz T, Martinez-Lozano C, Tomas V, Ibarra I (1992) Flow injection fluorimetric determination of thiamine and copper based on the formation of thiochrome. Talanta 39:907–911
Yang XF, Wang LP, Xu HM, Zhao ML (2009) A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide in aqueous solution. Anal Chim Acta 631:91–95
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21705056), the Natural Science Foundation of Shandong Province (ZR2017MB022, ZR2018BB057 and ZR2018 PB009) and the start-up funding from University of Jinan (511-1009408, 511-1009424).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 3398 kb)
Rights and permissions
About this article
Cite this article
Ni, P., Chen, C., Jiang, Y. et al. Fluorometric determination of sulfide ions via its inhibitory effect on the oxidation of thiamine by Cu(II) ions. Microchim Acta 185, 362 (2018). https://doi.org/10.1007/s00604-018-2906-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2906-3