Abstract
A method is described for the matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF)-based determination of benzo[a]pyrene (BaP) by using the metal-organic framework MIL-101(Fe) as a matrix. Following optimization of the experimental parameters, the method has a detection limit as low as 0.1 μg·L−1, which makes it more sensitive than previous methods for BaP analysis, and its analysis time is only 1 min. Its applicability was evaluated by analyzing sesame oil, linseed oil, camellia seed oil, and olive oil spiked with BaP at three levels (10, 1, and 0.5 μg·kg−1), and the recovery range was found to range from 80.0 to 114.8% with relative standard deviations (RSDs) between 3.9 and 13.7%.

The metal-organic framework MIL-101(Fe) was applied as a new matrix to the quantitative detection of benzo[a]pyrene (BaP) by matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are formed as a result of the incomplete combustion of oil, gasoline, wood, coal, and solid waste. Benzo[a]pyrene (BaP) has been used as a marker to study the occurrence and effects of carcinogenic PAHs in food [1] because of its presence in many food items, and despite being chemically inert, its metabolites are carcinogenic [2]. Many analytical methods have been developed for detection of BaP, including high-performance liquid chromatography [3], gas chromatography mass spectrometry [4] and fluorometry [5]. Although these methods are sensitive and accurate, they require complicated sample pretreatment and are slow. Therefore, a method for rapid detection of BaP is required.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), which is a soft ionization technique, has become a very important method because of its high-throughput and sensitivity. In contrast to conventional techniques such as GC-MS and HPLC-FLD, MALDI analysis has the advantages of a short experimental cycle and low consumption of samples (1 μL is sufficient). In addition, it does not require complex pretreatment. Consequently, it has been widely being used in the analysis of oligonucleotides, proteins, glycans, peptides, and lipids [6]. Generally, MALDI-TOF MS is difficult to apply to the detection of small molecules (< 600 Da) because the matrices in low molecular weight (LMW) analysis produce strong matrix ion peaks.
In the past decade, new matrices have been developed for MALDI-TOF MS of LMW compounds. These matrices include inorganic materials that do not ionize and produce little background interference. Metallic oxide graphene [7], mesoporous silica [8], carbon nanotubes [9], and fullerene [10] have been used as matrices for the analysis of LMW compounds. For example, Kim and co-workers reported the application of TiO2 nanowires to the detection of antibiotics in dairy milk samples [11]. The specific surface areas and electronic transport abilities of inorganic materials make them suitable for use as MALDI matrices. However, upon laser irradiation, inorganic materials can be easily lost from the target plate and contaminate the ion source.
Organic molecules such as metal phthalocyanines have also been used as MALDI matrices to detect LMW compounds. Zhang and co-workers successfully used them in the analysis of peptides, fatty acids, and phenols [12]. Although these organic molecules [13,15,15] are suitable for small molecule analysis, their syntheses are time consuming. An alternative class of compounds for this application is room temperature ionic liquids (RTILs) [16]. Li and Gross have tested RTILs as MALDI matrices for the quantification of oligodeoxynucleotides, peptides, and small proteins. Compared with the traditional solid matrices, RTILs show higher spot homogeneity and reproducibility because of their high solubilizing power, low vapor pressure, and broad liquid-phase temperature range [17]. However, RTILs have mainly been used to detect carbohydrates, alkaloids, nucleotides, and peptides and have rarely been applied to hazardous substances in food. Appropriate matrices are still required for analyzing hazardous LMW compounds.
Metal-organic frameworks (MOFs) are porous materials constructed from inorganic metal ions and organic ligands by self-assembly [18]. MOFs had been used in adsorption, gas storage, catalysis, drug delivery, and luminescence [19,21,21] because of their large surface areas and high porosity. Shih investigated different MOFs as matrices for surface-assisted laser desorption/ionization mass spectrometry to detect PAHs and found they showed high ionization capacity and provided stable and reproducible results [22].
Compared to other MOFs, MIL-101 has good cell dimensions (702,000 Å3), pore sizes (29–34 Å), and surface area (5900 m2 g−1) [23]. The inorganic metal ions present in MIL-101(Fe) generate coordinatively unsaturated metal sites (CUS), while BaP possesses many π electrons; thus, MIL-101(Fe) can form a stable mixture with BaP. Because the absorption capability of MIL-101(Fe) is strong, it can transfer energy to the analyte. As a result, MIL-101(Fe) was employed as a matrix for analysis of BaP in this study.
Experimental
Materials and reagents
α-Cyano-4-hydroxycinnamic acid (CHCA), 2,5-dihydroxybenzoic acid (DHB), sinapic acid (SA), and BaP were purchased from Sigma-Aldrich (MO, USA, www.sigma-aldrich.com). FeCl3.6H2O and trifluoroacetic acid (TFA) were purchased from Tianjin Chemical Reagent Factory (Tianjin, China, www.sjyc.company.lookchem.cn). Terephthalic acid and N,N-dimethylformamide were purchased from Chinese Medicine Group Chemical Reagent Co., Ltd. (Shanghai, China, http://www.sinoreagent.com/). Distilled water was obtained using a Milli-Q system (MA, USA, http://www.emdmillipore.com/US/en). Ethanol, methanol, and acetonitrile were of high-performance liquid chromatography grade, and all other reagents were of analytical grade. Vegetable oil samples were purchased at JD Mall (www.jd.com).
Instrumentation
An ultraviolet-visible spectrometer (Evolution 300, Thermo Fisher Scientific, Waltham, MA, www.thermofisher.com), a Fourier transform infrared spectrometer (Vector 22, Bruker Daltonik GmBH Life Sciences, Bremen, Germany, https://www.bruker.com/cn.html), and an X-ray powder diffractometer (D8 Advance, Bruker, Germany, https://www.bruker.com/cn.html) were used for the analysis of the samples. Scanning electron microscopy (SU1510, HITACHI, Japan, http://www.hitachi.com.cn/) was also employed.
MALDI-TOF MS analysis
All measurements were carried out using a Bruker UltrafleXtreme™ time-of-flight mass spectrometer (Bruker Daltonik GmBH Life Sciences, Bremen, Germany, https://www.bruker.com/cn.html) equipped with a nitrogen laser (λ = 337 nm). The instrument was operated in positive ion reflectron mode. Spectra were recorded from the sum of 500 laser shots. The instrument was calibrated with CHCA for accurate mass measurements. A polished steel target plate was used as the MALDI substrate. Data analysis was accomplished using FlexAnalysis 3.4 (Bruker Daltonik GmBH Life Sciences). All mass spectra were recorded in the m/z range from 220 to 280.
Preparation of the MOFs
MIL-101(Fe) was synthesized by the solvothermal method according to a previous report [24].
Preparation of MALDI-TOF sample
MIL-101(Fe) (6 mg) was dispersed in 1 mL of a mixed solution of ethanol and 0.1% TFA (1:1, v/v) by sonication for 5 min. An aliquot (1 μL) of the suspension was pipetted onto the target plate quickly, and the target was left at room temperature for 5–10 min to allow the suspension to form a thin matrix layer. Then, 1 μL of a solution of the analyte was pipetted onto the matrix layer. After evaporation of the solvent, the prepared target plate was subjected to analysis by MALDI-TOF MS.
A saturated solution of CHCA and DHB was prepared in acetonitrile/0.1% TFA (3:7, v/v), and a saturated solution of SA was prepared in ethanol.
Real samples detection
Individual stock solutions (1 mg·mL−1) of BaP were prepared in acetonitrile and stored in a refrigerator (−20 °C). Working standards (0.25–50 μg·L−1) were prepared by diluting an appropriate volume of the stock solution in acetonitrile.
Vegetable oil (1.5 g) was dissolved in 4 mL of acetonitrile/acetone (6:4, v/v), followed by centrifugation for 10 min and transfer of the supernatant to a new glass tube (15 mL). The extraction procedure was repeated three times, and the supernatants were combined and dried under a stream of nitrogen at 40 °C. The residue was reconstituted in 2 mL of n-hexane. Finally, 1 μL of the suspension of MIL-101(Fe) was pipetted onto the target plate, which was then left at room temperature to evaporate the solvent and form a thin film. Then, 1 μL of the analyte solution was dropped onto the matrix layer for subsequent analysis by MALDI-TOF MS.
Results and discussion
Choice of materials
MIL-101(Fe) provides high absorption capability in the UV-visible range, which meant that the material would be compatible with a standard MALDI-TOF MS equipped with a nitrogen laser. The iron in MIL-101(Fe) can produce coordinatively unsaturated metal sites of mixed valence iron (Fe2+/Fe3+), while BaP possesses many π electrons; thus, MIL-101(Fe) can form a stable mixture with BaP. Compared to other MOFs, MIL-101(Fe) has good cell dimensions (702,000 Å3), pore sizes (29–34 Å), and surface area (5900 m2 g−1). All these characteristics imply that it has superior potential for use as new matrix for MALDI-TOF MS.
Characterization of the MIL-101(Fe)
The ultraviolet-visible absorption peak for MIL-101(Fe) appeared between 300 and 400 nm and was attributed to the carboxyl groups (Fig. 1). This indicated that the material would be compatible with a standard MALDI-TOF MS equipped with a nitrogen laser.
The FT-IR spectrum of MIL-101(Fe) (Fig. S1A) showed peaks at 1600 cm−1, 1392 cm−1, and 552 cm−1, which were attributed to the asymmetrical stretching vibration, symmetrical stretching vibration, and bending vibration of the carboxyl groups, respectively. These results indicated that carboxylic acids were present in the ligands of MIL-101(Fe). Peaks for C=C bonds (1504 cm−1) and the surface bending of C-H bonds (749 cm−1) were characteristic of the benzene ring.
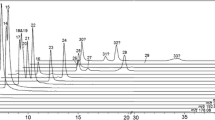
X-ray powder diffraction analysis of MIL-101(Fe) (Fig. S1B) showed large peaks at 10° and between 15° and 20°. These results were in accordance with the literature [30] and indicated that the synthesis of MIL-101(Fe) was successful.
Scanning electron microscopy of MIL-101(Fe) (Fig. 2) showed that MIL-101(Fe) was 1 μm in diameter, was a pyramid, and was homogeneous. The homogeneous MIL-101(Fe) was uniformly dispersed in the organic solvent after sonication and formed a homogeneous layer with the analyte. A homogeneous layer is beneficial for experimental repeatability. The simple structure of MIL-101(Fe) produces little background interference, which greatly enhances the ion signal. These characteristics suggest that MIL-101(Fe) will perform well as a matrix for MALDI- TOF MS.
MIL-101(Fe) as matrix for analysis of BaP
The suitability of MIL-101(Fe) for MALDI-TOF MS analysis was evaluated in comparison with the matrices CHCA, DHB, and SA. Mass spectra of BaP were obtained with some background interference when CHCA (Fig. 3a) and DHB (Fig. 3b) were used as the matrix but were almost not observed when SA was used as the matrix (Fig. 3c). Without any matrix, the ion signal of BaP can be detected (Fig. 3d) but with signal interference. With MIL-101(Fe), a strong ion signal was observed at 252.332 Da without background interference (Fig. 3e) and with a signal-to-noise ratio (S/N) up to 98 because the carboxylic acids in the ligands of MIL-101(Fe) can transmit protons for the desorption/ionization of BaP during laser irradiation. Moreover, the iron in MIL-101(Fe) can produce coordinatively unsaturated metal sites (CUS) of mixed valence iron (Fe2+/Fe3+) with thermal activation between 150 and 250 °C [25, 26]. In MALDI-TOF MS, laser irradiation can easily increase the temperature of the matrix to 200–250 °C [27]. BaP possesses many π electrons, and Fe3+ CUS can act as a Lewis acid site to induce interactions with BaP molecules (electron donors), while Fe2+ CUS can also establish further interactions with BaP through π back donation [26]. Therefore, MIL-101(Fe) can form a stable mixture with BaP, which would allow the matrix to deliver energy to the analyte more effectively.
The relative standard deviation (RSD, %, n = 3) for the signal intensity with MIL-101(Fe) was 5.3%, while the RSDs were above 15% when conventional matrices were used. Fig. S2 shows the surface morphologies of these matrices. The traditional organic matrices appear as lumpy crystals, whereas MIL-101(Fe) exhibits very homogenous co-crystallization of the matrix and analyte (Fig. S2D). Inhomogeneous morphology affects shot-to-shot reproducibility [13], which suggests that MIL-101(Fe) should provide excellent spot-to-spot reproducibility. The homogeneity of the MIL-101(Fe) matrix means that instead of having to find the optimum position for the laser, the sample can be analyzed at any point. Therefore, MIL-101(Fe) is suitable for quantitative analysis of BaP by MALDI-TOF MS.
Optimization of method
The following parameters were optimized: (A) dispersant for MIL-101(Fe), (B) concentration of MIL-101(Fe), (C) sample preparation protocol, (D) type of target plate, and (E) laser energy. Respective data and figures are given in the Electronic Supporting Material. We found the following experimental conditions to give the best results: (A) dispersant for MIL-101(Fe): ethanol/0.1% TFA (1:1, v/v), (B) concentration of MIL-101(Fe): 6 mg·mL−1, (C) sample preparation protocol: matrix/analyte sample preparation, (D) Type of target plate: polished steel, and (E) laser energy: 66%.
Validation of the method
To verify whether the MIL-101(Fe)-based MALDI-TOF MS method can be used for quantitative analysis, a series of experiments regarding the limit of detection (LOD), linear range (LR), and repeatability were performed under the optimized conditions. A series of BaP solutions at five concentration levels of 0.25, 1, 5, 10 and 50 μg·L−1 were prepared for the establishment of the calibration curve. For each concentration level, five replicate determinations were performed. As a result, a good linearity for BaP was observed in the concentration range of 0.25–50 μg L−1. The linear regression equation was Y = 52.63X + 148.34, with a determination coefficient (R2) above 0.99. Here, Y represents the signal intensity of the analyte, and X represents the concentration of the analyte (μg L−1). Based on a signal to-noise ratio of 3, the LOD was 0.1 μg L−1.
The repeatability of the method was evaluated by measuring the relative standard deviation (RSD) values acquired by conducting five parallel experiments at a concentration of 1 μg L−1. The RSD for BaP was 9.9%, indicating that the method has excellent stability and repeatability and can be used for quantitative analysis of BaP.
The method was compared with other reported method for the determination of BaP relative to LODs and analysis time (Table 1). As shown in Table 1, because the current MIL-101(Fe)-MALDI-TOF MS method is fast enough (only 1 min) and has low detection limits, it can meet the needs of the determination of BaP in complex samples. Application to the Detection of BaP in Vegetable Oil.
The method was applied to the analysis of BaP in four vegetable oil samples: sesame oil, linseed oil, camellia seed oil, and olive oil. As a result, BaP was detected in all the blank samples. The highest level detected was 3.1 μg·kg−1, which is lower than the maximum limit (< 5 μg·kg−1) set by the Codex Alimentarius Commission [32].
To validate the accuracy of the method, the four samples were spiked with standards of BaP at concentrations of 10, 1 and 0.5 μg·kg−1 and analyzed by the established method. Calculating the mean value of five duplicates, as listed in Table S1, showed that the recovery values were high (80.0–114.8%) and that the RSDs ranged from 3.9 to 13.7%. The mass spectra of the four vegetable oils are given in Fig. S4. These results reveal that the method can be applied to the analysis of real samples.
For real samples, extraction of linseed oil cannot be dried by nitrogen blow to result a little fat residue in redissolving, which will influence the quantitative accurate. Another the fat residue is not easy to dry on the target plate to affect the peak of target cause damage to the instrument. Therefore, it is necessary to develope a more effective sample extraction technique.
Conclusions
In summary, a MALDI-TOF MS method for the determination of BaP by using MIL-101(Fe) as the matrix was developed, and the method has excellent sensitivity. Compared with conventional matrices, such as CHCA, DHB, and SA, MIL-101(Fe) can give minimal background interference and can improve shot-to-shot reproducibility. The method has a wide range of linearity and good reproducibility, which can be used for quantitative analysis of actual samples, but the pretreatment of sample need to improve.
References
Zhang SX, Niu HY, Zhang YY, Liu JS, Shi YL, Zhang XL, Cai YQ (2012) Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatichydrocarbons from environmental water and milk samples. J Chromatogr A 1238:38–45
Roseiro LC, Gomes A, Santos C (2011) Influence of processing in the prevalence of polycyclic aromatic hydrocarbons in a Portuguese traditional meat product. Food Chem Toxicol 49:1340–1345
Wang R, Zl C (2017) A covalent organic framework-based magnetic sorbent for solid phase extraction of polycyclic aromatic hydrocarbons, and its hyphenation to HPLC for quantitation. Microchim Acta 184:3867–3874
Nacher-Mestre J, Serrano R, Portolés T, Berntssen MHG, Pérez-Sánchez J, Hernández F (2014) Screening of pesticides and polycyclic aromatic hydrocarbons in feeds and fish tissues by gas chromatography coupled to high-resolution mass spectrometry using atmospheric pressure chemical ionization. J Agric Food Chem 62:2165–2174
Garcia-Falcon MS, Simal-GaÂndara J, Carril-GonzaÂlez-Barros ST (2000) Analysis of benzo(a) pyrene in spiked fatty foods by second derivative synchronous specrtofluorimetry after microwave-assisted treatment of samples. Food Addit Contam 17:957–964
Su X, Zhou HY, Chen FC, Gao BX, Liu ZW, Zhang YH, Liu F, Liu F, Li ZR, Gao ZX (2013) Modified SBA-15 matrices for high-throughput screening of melamine in milk samples by MALDI-TOF MS. Int J Mass Spectrom 338:39–44
Dong XL, Cheng JS, Li JH, Wang YS (2010) Graphene as a novel matrix for the analysis of small molecules by MALDI-TOF MS. Anal Chem 82:6208–6214
Li XH, Wu X, Kim JM, Kim SS, Jin MS, Li DH (2009) MALDI-TOF-MS analysis of small molecules using modified mesoporous material SBA-15 as assisted matrix. J Am Soc Mass Spectrom 20:2167–2173
Tseng MC, Obena R, Lu YW, Lin PC, Lin PY, Yen YS, Lin JT, Huang LD, Lu KL, Lai LL, Lin CC, Chen YJ (2010) Dihydrobenzoic acid modified nanoparticle as a MALDI-TOF MS matrix for soft ionization and structure determination of small molecules with diverse structures. J Am Soc Mass Spectrom 21:1930–1939
Szabo Z, Vallant RM, Takatsy A, Bakry R, Najam-ul-Haq M, Rainer M, Huck CW, Bonn GK (2010) Laser desorption/ionization mass spectrometric analysis of small molecules using fullerene-derivatized silica as energy-absorbing material. J Mass Spectrom 45:45–552
Kim JI, Park JM, Noh JY, Hwang SJ, Kang MJ, Pyun JC (2016) Analysis of benzylpenicillin in milk using MALDI-TOF mass spectrometry with top-down synthesized TiO2 nanowires as the solid matrix. Chemosphere 143:64–70
Zhang S, Chen Y, Liu JA, Xiong SX, Wang GH, Chen J, Yang GQ (2009) New matrix of MALDI-TOF MS for analysis of small molecules. Chin Chem Lett 20:1495–1497
Yang HM, Wang JW, Song FR, Zhou YH, Liu SY (2011) Isoliquiritigenin (4,2′,4′-trihydroxychalcone): a new matrix-assisted laser desorption/ionization matrix with outstanding properties for the analysis of neutral oligosaccharides. Anal Chim Acta 701:45–51
Feng CH, CY L (2009) A new matrix for analyzing low molecular mass compounds and its application for determination of carcinogenic areca alkaloids by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Anal Chim Acta 649:230–235
He Q, Chen SM, Wang JN, Hou J, Wang JY, Xiong SX, Nie ZX (2013) 1-Naphthylhydrazine hydrochloride: a new matrix for the quantification of glucose and homogentisic acid in real samples by MALDI-TOF MS. Clin Chim Acta 420:94–98
Berthod A, Crank JA, Rundlett KL, Armstrong DW (2009) A second-generation ionic liquid matrix-assisted laser desorption/ionization matrix for effective mass spectrometric analysis of biodegradable polymers. Rapid Commun Mass Spectrom 23:3409–3422
Li YL, Gross ML (2004) Ionic-liquid matrices for quantitative analysis by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom 15:1833–1837
Liu YL, Zhao XJ, Yang XX, Li YF (2013) A nanosized metal-organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 138:4526–4531
Wang XM, Ma XM, Wang H, Huang PF, XZ D, XQ L (2017) A zinc(II) benzenetricarboxylate metal organic framework with unusual adsorption properties, and its application to the preconcentration of pesticides. Microchim Acta 184:3681–3687
Kayal S, Sun BC, Chakraborty A (2015) Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organicframeworks). Energy 91:772–781
Saedi Z, Safarifard V, Morsali A (2016) Dative and covalent-dative postsynthetic modification of a two-fold interpenetration pillared-layer MOF for heterogeneous catalysis: a comparison of catalytic activities and reusability. Microporous Mesoporous Mater 229:51–58
Shih YH, Chien CH, Singco B, Hsu CL, Lin CH, Huang HY (2013) Metal-organic frameworks: new matrixes for surface-assisted laser desorption/ionization mass spectrometry. Chem Commun 49:4929–4931
Férey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surblé S, Margiolaki I (2005) A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309:2040–2042
Maksimchuk NV, Kovalenko KA, Fedin VP, Kholdeeva OA (2012) Cyclohexane selective oxidation over metal–organic frameworks of MIL-101 family: superior catalytic activity and selectivity. Chem Commun 48:6812–6814
Wang DK, Huang RK, Liu WJ, Sun DR, Li ZH (2014) Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. ACS Catal 4:4254–4260
Yoon JW, Seo YK, Hwang YK, Chang JS, Leclerc H, Wuttke S, Bazin P, Vimont A, Daturi M, Bloh E, Llewellyn PL, Serre C, Horcajada P, Greneche JM, Rodrigues AE, Ferey G (2010) Controlled reducibility of a metal-organic framework with Coordinatively unsaturated sites for preferential gas sorption. Angew Chem Int Ed 49:5949–5952
Vimont A, Goupil JM, Lavalley JC, Daturi M, Surble S, Serre C, Millange F, Ferey G, Audebrand N (2006) Investigation of acid sites in a Zeotypic Giant pores chromium (III) carboxylate. J Am Chem Soc 128:3218–3227
Zhou RZ, Jiang J, Mao T, Zhao YS, Lu Y (2016) Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography-tandem mass spectrometry. Food Chem 207:43–50
Gosetti F, Chiuminatto U, Mazzucco E, Robotti E, Calabrese G, Gennaro MC, Marengo E (2011) Simultaneous determination of thirteen polycyclic aromatic hydrocarbons and twelve aldehydes in cooked food by an automated on-line solid-phase extraction-ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1218:6308
Li XS, JH W, LD X, Zhao Q, Luo YB, Yuan BF, Feng YQ (2011) A magnetite/oxidized carbon nanotube composite used as an adsorbent and a matrix of MALDI-TOF-MS for the determination of benzoapyrenew. Chem Commun 47:9816–9818
Zhang J, Dong XL, Cheng JS, Li JH, Wang YS (2011) Efficient analysis of non-polar environmental contaminants by MALDI-TOF MS with graphene as matrix. J Am Soc Mass Spectrom 22:1294–1298
Alimentarius C (1999) Codex standard for named vegetable oils Codex Stan 210:1-13
Acknowledgements
We would like to thank the National Key R&D Program of China (No. 2016YFD0401202), the Special Fund for Grain-scientific Research in the Public Interest (201513006-04), and the National Key Technology R&D Program (No. 2015BAD17B03).
Author information
Authors and Affiliations
Contributions
All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 8.44 mb)
Rights and permissions
About this article
Cite this article
Wang, Jp., Wang, Y., Guo, X. et al. Matrix assisted laser desorption/ionization time-of-flight mass spectrometric determination of benzo[a]pyrene using a MIL-101(Fe) matrix. Microchim Acta 185, 175 (2018). https://doi.org/10.1007/s00604-017-2627-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2627-z