Abstract
Carbon dots doped with sulfur and nitrogen (S,N-CDs) were utilised to design a paper-stripe based fluorescent probe for the detection of bilirubin. The S,N-CDs were synthesized through a microwave assisted route by using citric acid as carbon source and L-cysteine as a source of nitrogen and sulfur. The S,N-CDs exhibit bright blue fluorescence emission with a peak at 452 nm. Fluorescence is quenched by Fe(III) but selectively restored by bilirubin. The quenched fluorescent probe exhibit significant selectivity and sensitivity for bilirubin in the 0.2 nM to 2 nM concentration range, with a 0.12 nM detection limit. The method was applied to the determination of bilirubin in spiked human serum and urine samples. The method was used to design a paper based test stripe as a point of care device for visual bilirubin detection.

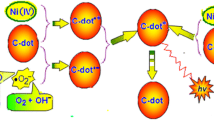
Schematic representation of sulphur and nitrogen doped carbon dots whose fluorescence is quenched by Fe(III) and turned on by bilirubin. Photograph of the corresponding system under day light and UV shows the feasibility of the phenomenon. The applicability of the assay was further extended by impregnating the probe on a filter paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal jaundice is recognized as one of the major healthcare issues facing by developing countries [1]. The accumulation of bilirubin (BR) in blood develops a condition called hyperbilirubinemia, when BR in blood plasma crosses 19.80 mg∙mL−1 in newborn and 1.19 × 10−2 mg∙mL−1 in adults [2]. BR is generated in human body as a result of heme catabolism and is excreted via bile. Yellow coloration of skin and sclera in association with jaundice is the symptom of elevated level of BR accumulation [3]. Toxic level of BR result in neurotoxicity in newborns which leads to neurodevelopmental abnormalities such as hearing loss, athetosis, rarely intellectual deficits and even extreme condition results in mortality or lifelong neurologic sequelae (kernicterus) [1]. Early diagnosis and treatment of hyperbilirubinemia is the only way to overcome this critical situation.
Available analytical methods to detect BR in serum sample are diazo methods, peroxidase methods, separation based methods and fluorometric methods [4]. Among these, fluorometric methods are simple, fast and easy. Fluorescent nanoprobes of metal nanoclusters, quantum dots and carbon dots (CDs) are currently employed for detecting various biomolecules [5, 6]. However, requirements of expensive precursors for metal nanoclusters and toxicity of quantum dots restricts its translation from bench side to bed side.
CDs are quasi-spherical nanoparticles with diameter less than 10 nm [7]. CDs received much attraction due to its cost-effective preparation, biocompatibility, low toxicity and optical properties [7]. They have potential applications in chemical sensing, nanomedicine, photo catalysis, electro catalysis, bioimaging and biosensing [7]. CDs can be synthesized through arc-discharge, laser ablation, electrochemical synthesis, thermal combustion, hydrothermal, template and microwave (MW) method [7]. Among these, MW method is fast and inexpensive method to synthesize CDs with good optical properties [7]. Heteroatom doping of CDs by Nitrogen, Sulfur, Boron and Phosphorous improves quantum yield by the creation of various surface functionalities and surface defects which favor fluorescence. Various methods to synthesize carbon dot that have been reported in the literature are correlated in Table S1.
CDs based fluorescence turn off detection of various metal ions like Hg(II), Fe(III), Co(II), Cr(VI) [8, 9], biomolecules like glucose [10] heparin [11] and reactive oxygen species like H2O2 [12] are reported in literature. Moreover, sensing mode of ON-OFF-ON for the detection of ascorbic acid, 4-nitrophenol, quercetin, 2, 4-dinitrophenol based on CDs have been explored [8, 13, 14]. Turn on detection is more effective compared to turn off detection as it is easy to detect a bright signal in dark background rather than a bright signal in a bright background [15].
In the present work, sulfur and nitrogen doped carbon dots (S,N-CDs) were prepared from citric acid (CA) and L-Cysteine. CA is an adaptable starting material for the synthesis of CDs by bottom-up routes due to various surface functionalities like –COOH, −OH and act as a carbon precursor [16,17,18]. L-Cysteine, a proteinogenic amino acid act as nitrogen and sulfur source [19]. Furthermore, it has antioxidant, anti-inflammatory properties and has the ability to neutralize heavy metals present in the body [19]. The fluorescent intensity of S,N-CDs can be significantly quenched by Fe(III) ions [20] and can be effectively restored by the addition of BR due to its tetrapyrrole ring structure which has an affinity towards Fe(III). It has been reported that Cu(II) quenched gold nanoclusters were employed for the detection of BR [2]. In this background, we tried to develop a CDs based low cost fluorescence turn on assay for BR. Turn on fluorescence analysis were performed in BR spiked human serum and urine samples. Practical applicability was further envisaged by designing paper based test stripe for direct detection of BR.
Experimental
Materials
L-Cysteine, Bilirubin and Hemoglobin were purchased from TCI, Tokyo, Japan (www.tcichemicals.com). Citric acid anhydrous Pure, Ferric chloride hexahydrate, Potassium chloride, Cholesterol extra pure, Ammonium Ferric Sulphate decahydrate, Silver Nitrate, Zinc Chloride dry Purified and Ferrous Chloride tetrahydrate for analysis were brought from Merck Specialities Pvt. Ltd., Mumbai (www.merck.co.in). Quinine Sulphate and Glutathione (≥ 98.0%) were purchased from Sigma Aldrich (www.sigmaaldrich.com). Cobalt Sulphate and Cadmium acetate were brought from Sisco Research Laboratories Pvt. Ltd. (www.srlchem.com). Ferric acetate and Manganese Chloride (97.0%) from Otto chemie Pvt. Ltd. (www.ottokemi.com). Copper Sulphate, Sodium Chloride, Calcium Nitrate, Magnesium sulphate, Urea, Acetone and Mercuric Chloride (98.0%) were brought from NICE chemicals Pvt. Ltd., Kochi (www.nicechemicals.com). Nickel Chloride hexahydrate Pure, D (−)-Fructose, D-Galactose extrapure, D-Glucose anhydrous were purchased from SRL Pvt. Ltd., Mumbai (www.srlchem.com). Sodium Hydroxide, Creatine and Uric Acid Stock Standard Solution (0.1%) were brought from Spectrum chemicals, India (www.spectrumchemical.com).
Instruments
The room temperature fluorescence measurements of all samples were studied using Jasco FP–750 spectroflurometer. High Resolution TEM images of S,N-CDs were taken in a JEOL JEM 2100 multipurpose, 200 kv analytical electron microscope. DLS measurements were carried out in HORIBA Scientific nano partica nanoparticles analyser SZ-100. The samples were analyzed in UV–Visible spectroscopic study using Perkin Elmer Lamda 365 UV Visible spectrophotometer. The functional group analysis is carried out using ATR-FTIR is performed using Agilent technologies Cary 630 FTIR spectrometer. Digital pH meter MK VI of systronics was used to measure the pH. The samples were prepared by microwave irradiation using POWER SOLO 17D, ONIDA instrument. Fluorescence lifetimes of all samples were carried out in Fluorolog Fluorescence Spectrophotometer, Horiba, USA using time-correlated single photon counting (TCSPC).
Synthesis of fluorescent S,N-CDs
CDs were synthesized via microwave irradiation method [21]. Citric acid anhydrous (0.2 g) was mixed with L-cysteine (0.4 g) in 4.0 mL water. Then, it was irradiated at 700 W for 40 s in a microwave oven. A thick syrupy colorless liquid obtained was diluted to 3.0 mL and the concentration becomes 3.902 μg∙mL−1. A dialysis membrane (MWCO: 1KDa); was then used to separate the S,N-CDs from any residual unreacted species.
Fe(III) modulated S,N-CDs
The stock solutions of Na(I), K(I), Mg(II), Ca(II), Cr(III), Mn(II), Fe(III), Fe(II), Co(II), Ni(II), Cu(II), Ag(I), Zn(II), Cd(II) and Hg(II) were prepared with 50 mM concentrations. In a typical assay, 20 μL of metal ion solution was mixed with 3.0 mL of S,N-CDs at pH 2.5 in room temperature before spectral measurements. The sensitivity was confirmed by adding serial concentrations of Fe(III) and all other metal ions in a similar way. The PL spectra were obtained at an excitation wavelength of 375 nm.
Detection of bilirubin
The stock solutions of bilirubin were prepared by taking 0.146 g bilirubin, add 20 μL 1 M NaOH to dissolve and made up to 15 mL by adding distilled water. S,N-CDs was semi quenched with Fe(III) [0.98 mM]. The sensitivity was studied by adding 20 μL of 3.80 nM of bilirubin in a sequential manner. All biomolecules (glucose, fructose, glutathione, galactose, cholesterol, urea, uric acid, creatine, hemoglobin) and bilirubin were studied in the same manner.
Real sample assay
Serum samples were collected from healthy volunteers in morning without EDTA stabilization as it may interfere with the turn on response of bilirubin towards Fe(III) quenched S,N-CDs. Then, it was spiked with bilirubin and diluted 10, 100, 1000 times, before analysis. Concentration of bilirubin spiked in 10 times diluted urine sample = 0.27 μM (critical concentration of bilirubin in hyperbilirubinemia condition) which was further diluted to 100 and 1000 times. Concentration of bilirubin spiked in serum sample = 340 μM (elevated concentration of bilirubin in newborn) and 170 μM (elevated concentration of bilirubin in adult) which was again diluted to 10, 100 and 1000 times.
Paper based test stripe assay
Whatmann No. 40 filter paper was used for the detection of bilirubin with a dimension of 8 × 1 cm. It was soaked with S,N-CDs and then dried. This S,N-CDs impregnated paper stripe was further soaked with different concentration of Fe(III) (1 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM) and again dried. Then, different concentration of bilirubin (0.33 μM, 0.66 μM, 0.99 μM, 1.33 μM, 1.66 μM, 1.99 μM) was added to the semi quenched system (5 mM of Fe(III)). Fluorescence is visualized under UV illumination of 366 nm.
Results and discussion
Synthesis and characterisation of S,N-CDs
S,N-CDs synthesized via MW mediated method in contrast to the previously reported hydrothermal technique [21]. Experimental conditions for MW synthesis were optimized to tune the fluorescence of S,N-CDs. Three factors such as molar ratio of the precursors, MW irradiation power and time of irradiation were optimized (Fig. S1-S2). At the optimum conditions (700 W, 40 s) colorless highly viscous liquid was formed due to the simultaneous formation of carbonized nanoparticles and citrazinic acid (molecular fluorophore) derivatives (Fig. S3, S4) [22]. The fluorescent S,N-CDs was again diluted with 3.0 mL water to perform further characterizations. The resulted fluorophores of citrazinic acid derivatives attached to the surface and interiors of carbon backbone was expected to elicit fluorescence. Maximum fluorescence emission for S,N-CDs was obtained with MW irradiation of 700 W for 40 s at 452 nm. At MW power higher than this, carbonization process predominates over fluorophore formation resulting in a charred mass. The concentration of this optimized S,N-CDs [S,N-CDs] was calculated to be 3.902 μg∙mL−1.
In order to get an idea about morphology and size distribution, High Resolution Transmission Electron Microscopic (HRTEM) characterization was carried out. HRTEM image reveals that the synthesized S,N-CDs are spherical in shape with a size range of 6 ± 1.5 nm (Fig. 1b and c). The Selected Area Electron Diffraction (SAED) pattern (inset of Fig. 1b) of S,N-CDs reveals that crystalline entities are embedded in an amorphous matrix. Dynamic Light Scattering (DLS) measurements of S,N-CDs provides a hydrodynamic diameter value of 47 ± 7 nm (Fig. S5) which was significantly larger than values from TEM images indicating the self assembly of citric acid based carbon dots into aggregates in polar solvents like water. The maximum fluorescence emission peak was observed at 452 nm upon the excitation of 375 nm and this wavelength is fixed for further studies (Fig. S6).
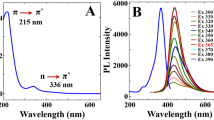
UV/Vis absorption spectrum of S,N-CDs shows an absorption peak at 352 nm, which can be assigned to n → π* transition of C = O functionalities present in S,N-CDs (Fig. S7) similar to that of the transitions in citrazinic acid [22]. The UV/Vis absorption band corresponding to the π → π* transitions are very weak. S,N-CDs shows bright blue fluorescence under UV illumination of 366 nm. Interestingly, on applying different excitation wavelength from 300 to 425 nm, the emission peak remains constant (Fig. 2a). This excitation independent emission behavior of CDs obey Kasha-Vavilov rule similar to citrazinic acid [23]. Disparity exists in literature regarding obeyance and disobeyance of Kasha-Vavilov rule in the luminescence of CDs [7, 20, 23, 24]. This elusive nature of S,N-CDs can be accorded by the different precursors and synthetic conditions adopted. The quantum yield of S,N-CDs was obtained as 78 ± 10% (Table S2).
a Fluorescence spectra of S,N-CDs at different excitation wavelengths ranging from 300 to 425 nm, pH -2.5, [S,N-CDs] = 3.902 μg∙mL−1. b ATR-FTIR spectrum of S,N-CDs at synthesized, pH -2.5. c Fluorescence emission spectra of S,N-CDs at various pH conditions: 1, 2.5, 3, 5, 7, 9, 11. d Fluorescence response of S,N-CDs with increasing ionic strength; ([KCl] = 0.0163 M - 0.25 M), pH -2.5, λex = 375 nm
ATR-FTIR spectra was used to identify the surface functionalities present on S,N-CDs. In ATR technique the absorption of carbon sample becomes greater with deeper light penetration at longer wavelength. Carbon sample displays absorption over the entire range from 4000 to 400 cm−1. This causes the observed base line dip towards the right end of the spectrum. The broad band at 3401 cm−1 represents stretching vibration of O-H and N-H respectively. The peak at 2563 cm−1 represents the stretching vibration of S-H [21]. A strong signal at 1702 cm−1 in S,N-CDs is due to the C = O stretching vibration of α,β-unsaturated carboxylic acid moieties, which is present in citrazinic acid and its derivatives [22]. The peak at 1616 cm−1 was attributed to C = C stretching vibrations [21]. The peaks at 1911 cm−1 and 1393 cm−1 are assigned to C-H bending vibration of aromatic compound and O-H bending vibration of phenol [22]. The peak at about 1054 cm−1 is due to the stretching vibration of C-N (Fig. 2b) [21]. These observations attest the existence of citrazinic acid derivatives in carbon dot framework.
The mechanism underlying the origin of fluorescence in CDs is still not certain. One of the widely accepted mechanisms is based on quantum confinement effect of CDs [8]. It has also been reported that defective surface states are the dominant factor for controlling fluorescence behavior of CDs compared to quantum size effect [17, 25]. CDs have high sp2 character with plenty of isolated π conjugate domains which in turn favors quantum confinement effect and band gap transitions to cause electron-hole pair recombination [8]. The second theory supports the defective state emission associated with surface defects [8]. CDs resemble graphene oxide as it symbolizes graphite nanocrystals with high heteroatom content [26]. The nitrogen linkage, disorder the carbon hexagonal ring and produce emissive energy traps. The sulphur atom also creates emissive energy traps. For sulphur and nitrogen doped carbon dots, the introduction of sulphur atom can enhance the effect of nitrogen atom. [27]. The molecular origin of fluorescence in the citric acid based CDs has also been attributed to the presence of derivatives of citrazinic acid [22]. Citric acid contains three carboxyl functionalities, which undergo intramolecular condensation and cyclisation with β-amino thiols like cysteine to form derivatives of citrazinic acid [22].
For further applications, the influence of pH towards the fluorescence intensity of S,N-CDs was monitored (Fig. 2c). For a wide range of pH values from 1 to 11, the fluorescence intensity of S,N-CDs increases with the decrease of pH and maximum fluorescence was observed at synthesized pH 2.5. The pH dependent fluorescence of S,N-CDs was due to the three possible prototropic equlibria, phenol ↔ phenolate, carboxylic ↔ carboxylate and ammonium ↔ amine ion that occurs in S,N-CDs [28]. The n → π* transition gains much predominance in polar protic medium where π → π* transitions are very weak. The maximum fluorescence was observed at acidic pH due to the much stabilization of n → π* transitions in aqueous polar medium than in non-polar medium. The shelf life of S,N-CDs was monitored by storing it at 4 °C for 37 days and fluorescence intensity is found to remain stable (Fig. S8). The stability in KCl solution was checked and found that the fluorescence intensity of S,N-CDs shows a slight decreasing trend with the increase in ionic strength (Fig. 2d). Increasing ionic strength may cause aggregation of S,N-CDs and restricting its fluorescence. The average fluorescence lifetime of S,N-CDs was obtained as 7.8 ± 0.2 ns (Fig. S9). The fluorescence decay curve of S,N-CDs is fitted in a tri-exponential function consisting of 3.97 ns (4.15%), 7.94 ns (96.83%) and 15.8 ns (−0.98%) contributing components.
Turn off phenomena in S,N-CDs
The fluorescence response of S,N-CDs with sequential addition of Fe(III) was investigated. As demonstrated in Fig. 3a, upon addition of Fe(III), the blue fluorescence intensity continuously decreased and the emission peak get slightly red shifted to 457 nm. The Stern-Volmer plot depicting fluorescence quenching effect on S,N-CDs by the addition of Fe(III) from 0.066 mM to 0.384 mM; (I0/I) = 0.3022 c + 1.09738; (R2 = 0.98273), where c is the concentration of Fe(III) (mol/L), I0 is the fluorescence intensity of S,N-CDs and I is that of quenched S,N-CDs by the addition of Fe(III) (Fig. 3b). The detection limit of Fe(III) was obtained as 23.08 μM (based on 3σ/slope, σ is the standard deviation) and the quenching efficiency of Fe(III) was found to be 76.22%.
a Fluorescence response of S,N-CDs upon the addition of various concentrations of Fe(III). ([Fe(III)] = 0 mM - 3.1250 mM), pH -2.5, λex = 375 nm. b The Stern-Volmer plot revealing linear fluorescence quenching of S,N-CDs by the addition of Fe(III); I0 is the initial fluorescence intensity of S,N-CDs and I is the fluorescence intensity after the addition of Fe(III). λex = 375 nm; [Fe(III)] = 0.066 mM to 0.384 mM. c The time resolved fluorescence decay profile of S,N-CDs (red) and Fe(III) quenched with bilirubin (blue); λex = 330 nm, λem = 452 nm ([Fe(III)] in quenched S,N-CDs = 1.61 mM). d The effect of various metal ions on the fluorescence response of S,N-CDs at pH 2.5; I0 is the initial fluorescence intensity of S,N-CDs and I is the fluorescence intensity of S,N-CDs after the addition of corresponding metal ions. (Inset: the fluorescence spectra showing the effect of different metal ions on S,N-CDs), ([metal ions] = 0.33 mM)
Fe(III) induced fluorescence quenching was monitored over the pH range from 1 to 11. The maximum quenching was obtained at 2.5 (Fig. S10) and this pH was taken for further investigations. In acidic medium, the phenolic groups present in citrazinic acid derivatives have more affinity towards Fe(III). As shown in Fig. 3c, the decay curve of S,N-CDs/Fe(III) shows a tri-exponential pathway consisting of 3.82 ns (4.36%), 7.65 ns (97.06%) and 15.30 ns (−1.42%) similar to that of S,N-CDs. The average fluorescence lifetime of S,N-CDs/Fe(III) was about 7.6 ± 0.4 ns.
Generally, the possible quenching mechanisms manifesting in CDs are static quenching, dynamic quenching, Förster resonance energy transfer (FRET), photo induced electron transfer (PET), inner filter effect (IFE) [29] and aggregation induced quenching [30]. The ratio of average fluorescence lifetime of S,N-CDs (τ0 = 7.8 ± 0.2 ns) to S,N-CDs/Fe(III) (τ = 7.6 ± 0.4 ns) was approximately unity (τ 0 / τ ≈ 1) and such consistency in fluorescence lifetime of two systems supports static quenching mechanism. Thus, possibility of dynamic, FRET and PET quenching mechanisms can be sidelined because such quenching mechanisms must be accompanied by significant change in fluorescent lifetime between S,N-CDs and turn off system. Static quenching occurs by the formation of non fluorescent ground-state complex through the interaction between S,N-CDs and Fe(III) [31]. Fe(III) absorbs and reacts with phenolic -OH, −COOH and –NH2 groups present on the surface of S,N-CDs leading to chelation or co-ordination. These complexations facilitate non-radiative electron-hole recombination leading to fluorescence quenching. Moreover, DLS measurements indicated that average diameter of S,N-CDs/Fe(III) is 358 ± 35 nm (Fig. S5) which is much higher than average diameter of S,N-CDs (47 ± 7 nm). This is an indication of the possibility of aggregation induced quenching between S,N-CDs and Fe(III). In addition, a minor contribution from IFE was also observed by the marginal overlapping between excitation spectrum of S,N-CDs with absorption spectrum of Fe(III) (Fig. S11). IFE is considered as an inevitable evil in quenching process associated with every fluorescence measurements due to attenuation of the excitation beam or absorption of emitted radiation by an excess concentration of S,N-CDs or by the quencher Fe(III) in solution [31]. Moreover, high ferromagnetic nature of Fe(III) may further split the discrete energy levels in S,N-CDs leading to overlap of energy levels facilitating fluorescence quenching [15]. The possibility of this latent phenomenon has to be investigated further as it was observed that Fe(III) acts as an universal fluorescent quencher of fluorescent nanostructures, such as quantum dots, metal nanoclusters and CDs [32,33,34].
To investigate the selectivity of S,N-CDs towards Fe(III) over other metal cations, quenching ability of representative cations (Cu(II), Ag(I), Zn(II), Cd(II), Hg(II), Na(I), K(I), Fe(II), Co(II), Ni(II), Mg(II), Ca(II), Cr(III) and Mn(II)) were evaluated (Fig. 3d, Fig. S12). The fluorescence response was measured under the same conditions that have been studied for Fe(III). As illustrated in Fig. 3d, the maximum fluorescence quenching was obtained for Fe(III) followed by Ag(I) and Cu(II). The redox potential of Fe(III), Ag(I) and Cu(II) are positive, so these ions facilitates electron transfer induced quenching. Eventhough Hg(II) possess high redox potential, but because of the closed d orbital present in Hg(II) inhibit its electron transfer induced quenching. The selectivity of the S,N-CDs towards Fe(III) over other metal cations was attributed by, a) the high electron affinity which in turn favors electron transfer, b) paramagnetic effect of Fe(III), c) high positive reduction potential of Fe(III). Eventhough Cr(III) and Mn(II) has half filled ‘d’ orbital, they have negative redox potential.The quenching effect of KCl solution (Fig. 2d) did not alter the selectivity or sensitivity of S,N-CDs/Fe(III) since the investigations were carried out at high concentrations. The effect of counter anions (Cl(I), SO4(II), CH3COO(I)) on the quenching ability of Fe(III) were studied (Fig. S13) and the results showed that fluorescence intensity remain unperturbed irrespective of the anions.
Turn on phenomenon in Fe(III) quenched S,N-CDs
Fluorescence response of Fe(III) quenched S,N-CDs towards BR was investigated by plotting II/I, were I is the ‘Turn On’ fluorescent intensity of Fe(III) quenched S,N-CDs and II is the fluorescent intensity of Fe(III) quenched S,N-CDs by the addition of BR. S,N-CDs is half quenched with an optimal concentration of Fe(III) 0.98 mM (S,N-CDs/Fe(III)) was employed as the fluorescent probe for further investigation. As expected, the addition of BR to the solution of S,N-CDs/Fe(III), restored its fluorescence (Fig. 4b). The plot depicting increase in fluorescence intensity of S,N-CDs/Fe(III) versus BR concentrations is linear over a concentration range of 0.2 nM to 2 nM with a limit of detection 0.12 nM (Fig. 4c). Turn on was studied over the entire concentration range from 2.18 pM to 0.21 μM and is shown in Fig. S14. The various present methods reported for bilirubin detection is compared in Table 1. Compared to the existing methods for BR detection, present work have detection limit as low as 0.12 nM. The fluorescent enhancement in presence of BR may be due to the stronger binding affinity between BR and Fe(III). BR consists of an open chain tetra pyrrole ring structure. It has anti-oxidant property [39] which can be utilized for the detection of BR. When BR comes into contact with S,N-CDs/Fe(III) an electron transfer reaction may takes place between the lone pair of nitrogen atoms present in the pyrrole ring of BR and Fe(III) enabling a complex formation between them. Furthermore, DLS measurements indicate that average diameter of turn on system is 116 ± 6 nm. This is much lower compared to average diameter of S,N-CDs/Fe(III) (358 ± 35 nm). This restoration in size confirms fluorescence turn on was due to the rupturing of Fe(III) mediated supramolecular aggregates of S,N-CDs. The maximum fluorescence recovery was observed at acidic pH (Fig. S15). Fluorescence lifetime of turn on system (7.7 ± 0.1 ns) is slightly higher compared to the lifetime of Fe(III) quenched S,N-CDs (7.6 ± 0.4 ns) and is in close agreement with the lifetime of S,N-CDs (7.8 ± 0.2 ns) establishing that regeneration of the fluorescence by the formation of free S,N-CDs (Fig. S16). BR detection was also carried out in S,N-CDs quenched with Ag(I) and Cu(II) and results shows that addition of BR to such quenched system creates further quenching rather than turn on establishing the selectivity of BR towards Fe(III) quenched S,N-CDs (Fig. S17). Furthermore, time dependent nature of fluorescent turn on phenomenon by BR towards the probe was investigated. Interestingly, it was observed that after standing for 10 min in daylight, turn on was increased due to photoisomerisation of BR (Fig. S18). Upon absorbing light from the blue region of the visible spectrum, one of the C = C double bonds (adjacent to five membered ring in BR) undergoes isomerization and the configuration changes from Z,Z-BR to Z,E-BR. (Fig. S19). Z,Z form of BR is indulged in internal hydrogen bonding and hence insoluble in water. But Z,E form of BR have more polar groups exposed to the environment rendering BR water solublility and exhibits more affinity towards Fe(III) [40].
a Schematic illustration of Turn On phenomena in Fe(III) quenched S,N-CDs (probe) using bilirubin. b The fluorescence Turn On response of bilirubin towards Fe(III) quenched S,N-CDs; ([Fe(III)] = 0.98 mM; [bilirubin] = 0–0.842 μM; pH = 2.5; λex = 375 nm). c Linear plot revealing fluorescence enhancement of Fe(III) quenched S,N-CDs (II/I) with increasing concentration of bilirubin; I and II are the fluorescence intensity before and after the addition of bilirubin to S,N-CDs quenched with 0.98 mM Fe(III). ([bilirubin] = 0.2 nM–2 nM). d Selectivity of Fe(III) quenched S,N-CDs probe with various biomolecules; ([biomolecules] = 0.1266 nM). e Sensitivity studies of Fe(III) quenched S,N-CDs probe with various biomolecules; ([biomolecules] = 0.1266 nM)
Selectivity and sensitivity
To investigate the selectivity of BR, several co-existing biomolecules such as glucose, fructose, galactose, glutathione, creatine, cholesterol, hemoglobin (Hb), urea and uric acid were analyzed [41]. Among these, BR induces a significant increase in fluorescence intensity (Fig. 4d). Sensitivity of BR towards the probe S,N-CDs/Fe(III) with these biomolecules were carried out (Fig. 4e, Fig. S20) and showed better fluorescence recovery even in presence of interfering biomolecules.
Real sample analysis
Performance of the probe was monitored in human urine. Urine sample of a person with clinical conditions such as chronic kidney disease (creatine level > 5.10 mM), hyperglycemia (glucose level > 9.15 mM) and uremic conditions (urea level > 0.15 M) may contain biomolecules in elevated concentration. So, it was necessary to compare the individual turn on response of these competing biomolecules with BR in aforementioned concentration and found out that turn on was maximum for BR (Fig. 5a). The figure depicts change in fluorescence intensities after and before BR addition (II-I) on S,N-CDs/Fe(III). The cumulative effect of urea, glucose and creatine towards S,N-CDs/Fe(III) was also studied. Even in the cumulative presence of these co-existing biomolecules, BR shows significant turn on (Fig. S21). Fluorescence recovery was studied for BR spiked water as well as in urine sample at different logarithmic dilutions (Fig. 5b) and successful results were obtained. However, small turn on signals created by co-existing biomolecules such as creatine, glucose, urea and Hb causes slight concern. These small false positive signals can be considered as a blessing in disguise as clinicians can cleverly correlate these signals arising from the presence of creatine, glucose and urea in urine sample as a complimentary diagnostic tool for hyperglycemia, chronic kidney disease and uremic conditions. Slight false positive signals arising from the presence of Hb can be correlated to the possibility of other clinical conditions such as urinary tract infections, bladder cancer and judiciously ruling out the possibility of non specific periodic appearance of Hb in urine samples. Further investigations have to be conducted in future to silence the signals created by these co-existing biomolecules. Detection of BR in human serum sample was also investigated. Appropriate dilutions of the sample (10, 100 and 1000 times) were used to detect BR at two different concentrations since the level of BR in hyperbilirubinemia condition is different for newborns (340 μM) and in adults (170 μM) [2]. A quantitative recovery of the spiked BR is obtained as shown in Fig. 5c, with a recovery of 84–116%. The data indicated that this fluorescent probe has good selectivity towards BR. Fig. 5d shows fluorescence of S,N-CDs, S,N-CDs/Fe(III), S,N-CDs/Fe(III)/BR under day light and UV light.
a Fluorescence recovery studies carried out in S,N-CDs quenched with 0.98 mM Fe(III) probe in urine sample spiked with bilirubin (0.276 μM), urea (0.1548 M), glucose (9.158 mM), creatine (5.109 mM) and hemoglobin (1 μM). b Change in fluorescence intensities after and before bilirubin addition on Fe(III) quenched S,N-CDs with bilirubin spiked urine sample at different dilutions (10, 100, 1000 times). ([bilirubin] = 0.276 μM). c Recovery percentage of bilirubin spiked serum sample at various dilutions (10, 100, 1000 times) ([bilirubin] of new born in hyperbilirubinemia condition = 340 μM (pink), [bilirubin] of adult in hyperbilirubinemia condition = 170 μM (blue)). d Photograph of cuvette containing (i) S,N-CDs under daylight, (ii) S,N-CDs under UV illumination (366 nm) (iii) S,N-CDs quenched with Fe(III) under daylight (iv) S,N-CDs quenched with Fe(III) under UV illumination (v) S,N-CDs quenched with Fe(III) and turn on with bilirubin under daylight (vi) S,N-CDs quenched with Fe(III) and turn on with BR under UV illumination. e Photograph under UV illumination (@ 366 nm) of filter paper strip impregnated with S,N-CDs and quenched with varying concentration of Fe(III) (i) 0 mM, (ii) 1 mM, iii) 5 mM, iv) 10 mM, v) 15 mM, vi) 20 mM, vii) 25 mM. f Photograph under UV illumination (@ 366 nm) of filter paper strip impregnated with S,N-CDs & soaked in 5 mM Fe(III) then dried and later turn on with varying concentration of bilirubin i) 0 μM ii) 0.332 μM iii) 0.665 μM iv) 0.998 μM v) 1.331 μM vi) 1.664 μM vii) 1.996 μM
Paper-stripe based detection
For practical application, a paper based test stripe has been designed. Whatmann no.40 filter paper was soaked in a solution containing S,N-CDs for 24 h and then dried. Under UV illumination (366 nm), the paper stripe shows blue emission. This paper stripe further soaked and dried in a solution containing 5 mM Fe(III) and the quenching of fluorescence was observed under UV illumination. The resulted filter paper was used as the probe for the detection of BR (Fig. 5e-f). Fluorescence turn on increases with increasing concentration of BR, and can be easily visualized under UV illumination (366 nm). These observations made it clear that paper based test stripe can be successfully used for the visual detection of BR.
Conclusion
In summary, we have demonstrated a turn on fluorescence assay for the detection of bilirubin using blue fluorescent S,N-CDs. The S,N-CDs was then characterized using HRTEM, DLS, ATR-FTIR, UV-Vis and TCSPC lifetime instruments. The fluorescence was quenched with ferric ion via a static quenching mechanism and was then restored by the addition of bilirubin with limit of detection in nanomolar range. Real sample assay faced slight individual interferences from coexisting biomolecules which was observed to be nullified by the cumulative presence of biomolecules. Furthermore, bilirubin detection was investigated in biological fluids such as serum and urine and showed good recovery percentage. The current work was utilized to develop paper based test stripe for detection of bilirubin. The insight gained from these results can be utilized for the future development of point of care diagnostic devices.
References
Tikmani SS, Warraich HJ, Abbasi F, Rizvi A, Darmstadt GL, Zaidi AK (2010) Incidence of neonatal hyperbilirubinemia: a population-based prospective study in Pakistan. Tropical Med Int Health TM & IH 15(5):502–507. https://doi.org/10.1111/j.1365-3156.2010.02496.x
Basu S, Sahoo AK, Paul A, Chattopadhyay A (2016) Thumb imprint based detection of hyperbilirubinemia using luminescent gold nanoclusters. Sci Rep 6:39005. https://doi.org/10.1038/srep39005 https://www.nature.com/articles/srep39005#supplementary-information
Maisels MJ (2015) Managing the jaundiced newborn: a persistent challenge. CMAJ 187(5):335–343. https://doi.org/10.1503/cmaj.122117
Santhosh M, Chinnadayyala SR, Kakoti A, Goswami P (2014) Selective and sensitive detection of free bilirubin in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens Bioelectron 59:370–376. https://doi.org/10.1016/j.bios.2014.04.003
Zhang JJ, Cheng FF, Li JJ, Zhu J-J, Lu Y (2016) Fluorescent nanoprobes for sensing and imaging of metal ions: recent advances and future perspectives. NanoToday 11:309–329. https://doi.org/10.1016/j.nantod.2016.05.010
Li JJ, Cheng FF, Huang H, Li L, Li J-J (2015) Nanomaterial based activatable imaging probes: from design to biological applications. Chem Soc Rev 44:7855. https://doi.org/10.1039/c4cs00476k
Xu Q, Kuang T, Liu Y, Cai L, Peng X, Sreenivasan Sreeprasad T, Zhao P, Yu Z, Li N (2016) Heteroatom-doped carbon dots: synthesis, characterization, properties, photoluminescence mechanism and biological applications. J Mater Chem B 4(45):7204–7219. https://doi.org/10.1039/C6TB02131J
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362–381. https://doi.org/10.1039/C4CS00269E
Miao X, Yan X, Dan Q, Li D, Tao FF, Sun Z (2017) Red emissive sulfur, nitrogen doped carbon dots and their application in ion detection and theranostics. ACS Appl Mater Interfaces 9:18549–18556. https://doi.org/10.1021/acsami.7b04514
Zohu X, Gao X, Liu M, Wang C, Chu F (2017) A poly (5-Indolylboronic acid) based molecular imprint doped with carbon dots for fluorometric determination of glucose. Microchim Acta 184:4175–4181. https://doi.org/10.1007/s00604-017-2448-0
Wang R, Wang X, Sun Y (2017) Aminophenol-based carbon dots with dual wavelength fluorescence emission for determination of heparin. Microchim Acta 184(1):187–193. https://doi.org/10.1007/s00604-016-2009-y
Yang H, Li F, Zou C, Huang Q, Chen D (2017) Sulfur-doped carbon quantum dots and derived 3D carbon nanoflowers are effective visible to near infrared fluorescent probes for hydrogen peroxide. Microchim Acta 184(7):2055–2062. https://doi.org/10.1007/s00604-017-2181-8
Dong Y, Wang R, Tian W, Chi Y, Chen G (2014) “Turn-on” fluorescent detection of cynide based on polyamine functionalized carbon quantum dots. RSC Adv 4:3701. https://doi.org/10.1039/c3ra45893h
Dai H, Shi Y, Wang Y, Sun Y, Hu J, Ni P, Li Z (2014) A carbon dot based biosensor for melamine detection by fluorescenc resonance energy transfer. Sensors Actuators B Chem 202:201–208. https://doi.org/10.1016/j.snb.2014.05.058
Anjali Devi JS, Salini S, Anulekshmi AH, Praveen GL, Sony G (2017) Fe (III) ion modulated l-DOPA protected gold nanocluster probe for fluorescence turn on sensing of ascorbic acid. Sensors Sens Actuators B Chem 246:943–951. https://doi.org/10.1016/j.snb.2017.02.151
Dhenadhayalan N, Lin K-C, Suresh R, Ramamurthy P (2016) Unravelling the multiple emissive states in citric-acid-derived carbon dots. J Phys Chem C 120(2):1252–1261. https://doi.org/10.1021/acs.jpcc.5b08516
Pan L, Sun S, Zhang A, Jiang K, Zhang L, Dong C, Huang Q, Wu A, Lin H (2015) Truly fluorescent excitation-dependent carbon dots and their applications in multicolor cellular imaging and multidimensional sensing. Adv Mater (Deerfield Beach, Fla) 27(47):7782–7787. https://doi.org/10.1002/adma.201503821
Margraf JT, Strauss V, Guldi DM, Clark T (2015) The electronic structure of amorphous carbon nanodots. J Phys Chem B 119(24):7258–7265. https://doi.org/10.1021/jp510620j
Yin J, Ren W, Yang G, Duan J, Huang X, Fang R, Li C, Li T, Yin Y, Hou Y, Kim SW, Wu G (2016) L-cysteine metabolism and its nutritional implications. Mol Nutr Food Res 60(1):134–146. https://doi.org/10.1002/mnfr.201500031
Chen Y, Wu Y, Weng B, Wang B, Li C (2016) Facile synthesis of nitrogen and sulfur co-doped carbon dots and application for Fe(III) ions detection and cell imaging. Sensors Actuators B Chem 223:689–696. https://doi.org/10.1016/j.snb.2015.09.081
Amjadi M, Manzoori JL, Hallaj T, Azizi N (2017) Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J Lumin 182:246–251. https://doi.org/10.1016/j.jlumin.2016.10.021
Schneider J, Reckmeier CJ, Xiong Y, von Seckendorff M, Susha AS, Kasák P, Rogach AL (2017) Molecular fluorescence in citric acid-based carbon dots. J Phys Chem C 121(3):2014–2022. https://doi.org/10.1021/acs.jpcc.6b12519
Liu Y, Zhou L, Li Y, Deng R, Zhang H (2017) Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nano 9(2):491–496. https://doi.org/10.1039/C6NR07123F
Zhang Y, He J (2015) Facile synthesis of S, N co-doped carbon dots and investigation of their photoluminescence properties. Phys Chem Chem Phys 17(31):20154–20159. https://doi.org/10.1039/C5CP03498A
Ding H, S-B Y, Wei J-S, Xiong H-M (2016) Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10(1):484–491. https://doi.org/10.1021/acsnano.5b05406
Hong G, Diao S, Antaris AL, Dai H (2015) Carbon Nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev 115(19):10816–10906. https://doi.org/10.1021/acs.chemrev.5b00008
Park Y, Yoo J, Lim B, Kwon W, Rhee SW (2016) Improving the functionality of carbon nanodots: doping and surface functionalization. J Mater Chem A 4(30):11582–11603. https://doi.org/10.1039/C6TA04813G
Dutta Choudhury S, Chethodil JM, Gharat PM, Praseetha PK, Pal H (2017) pH-elicited luminescence functionalities of carbon dots: mechanistic insights. J Phys Chem Lett 8(7):1389–1395. https://doi.org/10.1021/acs.jpclett.7b00153
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184(7):1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Yoosaf K, Ipe BI, Suresh CH, Thomas KG (2007) In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. J Phys Chem C 111(34):12839–12847. https://doi.org/10.1021/jp073923q
Li G, Fu H, Chen X, Gong P, Chen G, Xia L, Wang H, You J, Wu Y (2016) Facile and sensitive fluorescence sensing of alkaline phosphatase activity with Photoluminescent carbon dots based on inner filter effect. Anal Chem 88(5):2720–2726. https://doi.org/10.1021/acs.analchem.5b04193
Li S, Li Y, Cao J, Zhu J, Fan L, Li X (2014) Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal Chem 86(20):10201–10207. https://doi.org/10.1021/ac503183y
Tang T, Ouyang J, Hu L, Guo L, Yang M, Chen X (2016) Synthesis of peptide templated copper nanoclusters for fluorometric determination of Fe(III) in human serum. Microchim Acta 183(10):2831–2836. https://doi.org/10.1007/s00604-016-1935-z
Li G, Lv N, Bi W, Zhang J, Ni J (2016) Nitrogen-doped carbon dots as a fluorescence probe suitable for sensing Fe3+ under acidic conditions. New J Chem 40(12):10213–10218. https://doi.org/10.1039/C6NJ02088G
Hullin DA, McGrane MT (1991) Effect of bilirubin on uricase-peroxidase coupled reactions. Implications for urate measurement in clinical samples and external quality assessment schemes. Ann Clin Biochem 28(Pt 1):98–100. https://doi.org/10.1177/000456329102800117
Rand RN, Ad P (1962) A new Diazo method for the determination of bilirubin. Clin Chem 8(6):570–578
Santhosh M, Chinnadayyala SR, Singh NK, Goswami P (2016) Human serum albumin-stabilized gold nanoclusters act as an electron transfer bridge supporting specific electrocatalysis of bilirubin useful for biosensing applications. Bioelectrochemistry (Amsterdam, Netherlands) 111:7–14. https://doi.org/10.1016/j.bioelechem.2016.04.003
Senthilkumar T, Asha SK (2015) Selective and sensitive sensing of free bilirubin in human serum using water-soluble Polyfluorene as fluorescent probe. Macromolecules 48(11):3449–3461. https://doi.org/10.1021/acs.macromol.5b00043
Jansen T, Daiber A (2012) Direct antioxidant properties of bilirubin and Biliverdin. Is there a role for biliverdin reductase? Front Pharmacol 3:30. https://doi.org/10.3389/fphar.2012.00030
Fevery J (2008) Bilirubin in clinical practice: a review. Liver Int 28(5):592–605. https://doi.org/10.1111/j.1478-3231.2008.01716.x
Wu H, Jiang J, Gu X, Tong C (2017) Nitrogen and sulfur co-doped carbon quantum dots for highly selective and sensitive fluorescent detection of Fe(III) ions and L-cysteine. Microchim Acta 184(7):2291–2298. https://doi.org/10.1007/s00604-017-2201-8
Acknowledgements
The authors acknowledge the Professor and Head of the Department of Chemistry, University of Kerala for providing the research facilities. Authors also appreciate SAIF, MGU – India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2.50 mb)
Rights and permissions
About this article
Cite this article
Anjana, R.R., Anjali Devi, J.S., Jayasree, M. et al. S,N-doped carbon dots as a fluorescent probe for bilirubin. Microchim Acta 185, 11 (2018). https://doi.org/10.1007/s00604-017-2574-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2574-8