Abstract
The authors have discovered that vanadium disulfide (VS2) nanosheets, synthesized by a hydrothermal method, exert stable peroxidase-like activity. The catalytic activity, with H2O2 as a cosubstrate, follows Michaelis-Menten kinetics and varies with temperature, pH value and H2O2 concentration. Two-dimensional VS2 sheets acting as peroxidase (POx) mimics can replace horseradish peroxidase due to their availability, robustness, and reusability. The POx-like activity of VS2 sheets was exploited to design a colorimetric glucose assay by using 3,3′,5,5′-tetramethylbenzidine as a substrate and by working at an analytical wavelength of 652 nm. The assay covers the 5 to 250 μM glucose concentration range with a 1.5 μM detection limit. It was applied to the analysis of glucose in fruit juice. In our perception, the peroxidase-like nanozyme out of the family of transition metal dichalcogenides presented here has a wide scope in that it may stimulate promising biocatalytic applications in biotechnology and analytical chemistry.

Layered VS2 nanosheets were prepared via hydrothermal synthesis and are shown to exert superior peroxidase-mimicking activity. Using these POx nano-mimics, a sensitive colorimetric assay for glucose was developed and applied to fruit juice analysis. This work unlocks the access of VS2 to biocatalysis and bioassays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural enzymes, as traditional efficient and substrate-specific biocatalysts, have been practically applied in food processing, chemical industry, biomedical and biochemistry fields for decades [1]. Whereas, enzymes are usually composed of proteins (or RNAs), thus these high-cost biocatalysts are vulnerable to inactivation unless in the mild condition. To address these drawbacks, artificial enzymes have been developed as robust alternative for natural enzymes. The development of nanotechnology brings great power and application prospect to the research of enzyme mimetics, due to the intrinsic properties of nanomaterials such as large surface-to-volume ratio, high stability, low cost, biocompatibility, and tunable catalytic activity [2,3,4]. Up to date, many functional nanoscale materials (metal-, metal oxide-, carbon based- nanostructures, etc.) have been reported to imitate the catalysis of natural enzymes, playing key role in biosensor design and biosystem modulation. Developing novel biocatalytic-active nanomaterials (nanozymes) is currently forefront research in inorganic, biological and analytical chemistry.
Nanozymes with two-dimensional (2D) structure such as graphene oxide (GO) [5, 6], possessing high surface-to-volume ratio, can achieve a high load of catalytic substrate on a single nanosheet and provide the superiority of surface engineering strategies. Inspired by the success of GO in nanozyme research, graphene-like two-dimensional layered materials have attracted extensive attention in biocatalysis and biomedicine [7,8,9]. Vanadium disulfide (VS2) nanosheets, a representative of the 2D transition metal dichalcogenides (TMDs), have been star nanomaterial in electrochemistry [10], catalysis [11], and energy storage fields [12], due to their interesting properties gifted by unsaturated d-orbitals of transition metals. Coincidentally, vanadium can be found in many natural enzymes of biological systems. Generally vanadium is in close association with the active center or the cofactor in enzymes such as vanadium chloroperoxidase [13], vanadium bromoperoxidase [14], and vanadium nitrogenase [15]. Linking together the unique properties of 2D VS2 nanosheets and the richness of vanadium in natural enzymes, nanoscale VS2 is of great possibility to exert enzyme-like activity, switching on the access of VS2 to biocatalytic applications. However, to our knowledge the enzyme-like biocatalysis of VS2 has never been investigated before.
Diabetes mellitus is one of the most globally prevalent diseases, resulting in increasing disability, reduced life expectancy, and enormous healthcare expenses for almost every community [16]. Glucose is a marker for the diagnosis of diabetes, and excessive glucose intake is an important issue concerning the induction or exacerbation of diabetes [17]. Quantitative analysis of glucose in food samples can provide dietary guidelines for diabetic patients. Therefore, novel detection methods are demanded for detecting glucose in food. Among the detection methods for glucose, colorimetric glucose assays using nanozymes are particularly attractive because of their simple operation, low cost, and fast visual signal readout by the bare eye [18].
In this work, we report that layered VS2 nanosheets, one of the common TMDs, exhibit intrinsic peroxidase-like activity. They can catalyze the oxidation of a substrate in the presence of H2O2 to produce a colored reaction product. Moreover, VS2 nanosheets as POxX-like biocatalysts are demonstrated to exhibit good catalytic properties, stability, and reusability, rivaling many other peroxidase mimetics and natural horseradish peroxidase (HRP). The biocatalytic mechanism of VS2 POx mimics is also investigated in this work. Finally thanks to these findings, the novel use of such two-dimensional VS2 as readily available nanocatalysts is demonstrated for sensitive colorimetric detection of glucose in fruit juice.
Material and methods
Chemical and materials
Ammonium metavanadate (NH4VO3), thioacetamide (TAA), glucose, fructose, maltose, lactose, and sucrose were purchased from Aladdin chemistry Co. Ltd. (Shanghai, China, http://www.aladdin-e.com/). Glucose Oxidase (GOx, from Aspergillus Niger), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 3,3′,5,5′-tetramethylbenzidine (TMB), o-phenylenediamine (OPD), and Glucose (HK) Assay Kit (GAHK20) were purchased from Sigma Aldrich (Shanghai, China, http://www.sigmaaldrich.com/china-mainland.html). NH3•H2O, HAc, NaAc, NaOH and anhydrous ethanol were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China, http://www.sinoreagent.com/). Commercial apple juice and orange juice (Minute Maid, Coca-Cola Co., http://www.minutemaid.com/) were purchased from local supermarket. All of these reagents were analytical grade and used as received. Ultrapure water (18.2 MΩ·cm) produced by a Milli-Q system was used throughout this work.
Instruments and characterizations
Field emission scanning electron microscope (SEM) image was taken by an S-4800 (Hitachi, Japan, http://www.hitachi.com/). Powder X-ray diffraction (XRD) patterns were obtained using a powder diffractometer (Bruker D8 Advanced Diffractometer System, Germany, http://www.bruker.com/) with a Cu Kα (1.5418 Å) source. Raman spectra were recorded by a micro-Raman spectrometer (i-Raman Plus, B&W TEK Inc., USA, http://www.bwtek.com/). The UV-vis spectra were measured with a UV-2550 spectrophotometer (Shimadzu, Japan, http://www.shimadzu.com/). All pH measurements were performed with a PB-10 digital pH-meter (Sartorius, Germany, https://www.sartorius.com/) with a combined glass-calomel electrode.
Synthesis of VS2 nanosheets catalysts
VS2 nanosheets were obtained through a hydrothermal method. Briefly, 8 mmol NH4VO3 was dissolved in 72 mL NH3•H2O aqueous solution (VNH3•H2O: Vdeionized water is 1:11). Note that NH4VO3 should be completely dissolved in the solution before the next step. Then, 40 mmol thioacetamide (TAA) was added under magnetic stirring and this mixture was kept stirring for 30 min with the formation of a dark black solution. Subsequently, the solution was transferred into an autoclave and incubated at 180 °C for 24 h. After thehydrothermal treatment, fresh black precipitates were separated by centrifugation, and washed with deionized water and ethanol thoroughly for several times, respectively. The final product was dried at 40 °C in vacuum overnight, yielding the black powder samples denoted VS2 product.
Peroxidase-like catalysis of VS2 nanosheets
The time-dependent kinetics of VS2 nanosheets was studied as follows: A reaction system with different concentration of VS2 (from 0 to 80 μg·mL−1), 20 mM H2O2 and 0.5 mM TMB was monitored using a UV-2550 spectrophotometer in time scan mode at 652 nm right after all of the reagents were mixed. The operation of the leaching experiment is the same as the above, except that the VS2 solution is replaced with leaching solution. PH test was carried out at room temperature and the pH value of the reaction buffer was adjusted in the range of 2.0–7.0 by the addition of HCl or NaOH. Temperature test was conducted in acetate buffer (pH 4.0) in the range of 4–70 °C. The re-utilization experiment was carried out using a scale-up reaction system (1 mg VS2 used) and repeated the operation under identical reaction conditions. The ROS scavenging assays were carried out by adding different concentration of scavenger into the reaction system (50 μg·mL−1 VS2, 20 mM H2O2, and 1 mM TMB) and the UV-vis absorption was measured after 15 min incubation.
Kinetic study of VS2 as peroxidase mimetics
Unless otherwise stated, steady-state kinetic assays were carried out under standard reaction conditions (50 μg·mL−1 VS2 nanosheets at room temperature in pH 4.0 acetate buffer) by varying concentrations of TMB at a fixed concentration of H2O2 or vice versa. All the reactions were monitored in time scan mode at 652 nm using a Shimadzu UV-2550. Catalytic parameters were determined by fitting the absorbance data to the Michaelis-Menten equation (Eq. 1).
The Michaelis-Menten equation describes the relationship between the rates of substrate conversion by an enzyme and the concentration of the substrate. In this equation, v is the rate of conversion, v max is the maximum rate of conversion, [S] is the substrate concentration, and K m is the Michaelis constant.
H2O2 and glucose colorimetric detection assays
The H2O2 detection assay was conducted by adding increasing amount of H2O2 into the reaction system (50 μg·mL−1 VS2 and 1 mM TMB in 0.1 M acetate buffer, pH 4.0) and incubating for 10 min at room temperature. For the glucose calibration, 5 μL of 40 mg·mL−1 glucose oxidase (GOx) were mixed with 100 μL phosphate buffered solution (5 mM, pH 7.0) containing different amount of D-glucose and then incubated at 37 °C for 45 min. Subsequently, the glucose reaction solution was added into 400 μL acetate buffer (0.1 M, pH 4.0) containing 1 mM TMB and 50 μg·mL−1 VS2 nanosheets. After incubated at room temperature for another 10 min, the reaction solution was monitored by the UV-vis spectrophotometer. For glucose determination in fruit juice samples (apple juice and orange juice), the juices were firstly centrifuged at 12,000 rpm (7727 rcf) for 40 min to remove the precipitates. Then the supernatants were diluted 100-fold using 0.1 M NaAc buffer (pH 4.0) and stored overnight at room temperature before use. As a comparison, the glucose content in juice samples was also determined by a Glucose (HK) Assay Kit (Sigma Aldrich).
Results and discussion
Structure characterization of VS2 nanosheets
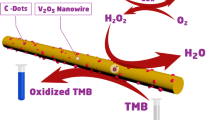
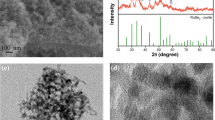
As schematically illustrated in Fig. 1a, layered VS2 nanosheets were synthesized via a hydrothermal method, using ammonium metavanadate (NH4VO3) and thioacetamide (TAA) as precursor materials. The XRD pattern of the VS2 nanosheets (before catalysis) is presented in Fig. 1b, in which all characteristic peaks are consistent with the standard card of VS2 (JCPDS NO. 89–1640). Figure 1c shows the Raman spectrum of the VS2 (before catalysis) in the range of 100–1100 cm−1. As reported previously, the Raman bands occurring at 140.4, 192.0, 282.0, 406.6, 687.8, and 993.2 cm−1 are assigned to the rocking and stretching vibrations of V–S bonds or their combination [19]. As the SEM image in Fig. 1d shows, the as-prepared VS2 exhibit nanosheet-like morphology with a diameter of 3–6 μm and a thickness of 50–100 nm. As typical two-dimensional nanostructures, VS2 nanosheets have great application potential in biocatalysis by employing surface engineering strategies, like their analogues (MoS2 and WS2) in TMDs family [20,21,22]. Finally, the chemical maps of the constituent elements V and S were obtained using the energy dispersive X-ray spectroscopy (EDX) technique. Figure 1e shows that V and S elements are homogeneously distributed with the ratio of about 1: 2. The structure characterization results above indicate the successful synthesis of two-dimensional layered VS2 nanosheets.
Catalytic properties of POx-like VS2 catalysts
Firstly we studied the catalytic oxidation ability of VS2 toward peroxidase substrate (ABTS, TMB, and OPD) to evaluate their availability as peroxidase mimics. As showed in Fig. 2a, negligible change can be seen in the UV-vis spectra (The dash lines) when VS2 was incubated only with peroxidase substrate. However, on addition of H2O2, colored oxidation products are formed that have characteristic absorption peaks (oxTMB: 652 nm, oxABTS: 420 nm; oxOPD: 450 nm). The results clearly demonstrate that vanadium disulfide can simulate the catalysis of horseradish peroxidase (HRP). TMB was used as the substrate in subsequent studies because of its acceptable signal generation under catalytic oxidation.
POx-like catalytic properties of VS2 nanosheets. a UV-vis absorption spectra recording the oxidation of 1 mM ABTS (green line), TMB (blue line), and OPD (yellow line) catalyzed by VS2 nanosheets (25 μg·mL−1) in the presence of H2O2 (20 mM). The corresponding dashed line indicates the incubation of VS2 with ABTS, TMB, or OPD only. Reaction time t = 10 min. b Time- and catalyst concentration-dependent absorbance at 652 nm measured from the reaction solutions containing 20 mM H2O2, 0.5 mM TMB, and VS2 nanosheets of different concentrations in 0.1 M acetate buffer (pH 4.0) at room temperature
Using the oxidation of TMB by H2O2 as a model reaction, the time-dependent kinetics of VS2 in different concentrations was monitored. As shown in Fig. 2b, the absorbance of oxTMB increases steadily with the increasing VS2 nanosheets and the time past. This indicates the catalytic reaction is dependent on VS2 concentration and time. Subsequently, the following catalytic conditions were optimized: (a) reaction pH value; (b) temperature. Respective data and illustrations are given in Fig. S1 and Fig. S2. For simplicity, 10 min reaction time, 50 μg•mL−1 VS2, and mild catalytic conditions (pH value of 4.0, at room temperature) were adopted as the standard conditions for subsequent studies.
Stability and reusability of VS2 catalysts
Normally, it is necessary to eliminate the possibility that the catalytic activity is induced by possible leaching ions from VS2 such as vanadate. As showed in Fig. S3, the leaching solution of 100 μg·mL−1 VS2 exhibits almost no activity compared to that of 100 μg·mL−1 VS2. It demonstrates that the catalytic activity is derived from VS2 nanosheets themselves rather than the leaching ions. In addition, to test the stability of the catalyst, the XRD pattern and Raman spectra of the catalyst after catalysis (Fig. 1b, c) were also obtained at first. No differences can be seen in the XRD and Raman data when comparing VS2 nanosheets before and after catalysis. This indicates that the catalyst is still present as vanadium disulfide after catalytic reaction. Further, the thermal stability of VS2 was examined by heating the catalysts at 60, 80, and 100 °C before catalytic reaction. Fig. S4 shows that although the catalyst loses ~25% activity after 1 h heating treatment at 100 °C, the catalyst still maintains 90% activity after treatment at 60 or 80 °C. This indicates an acceptable thermal stability of VS2 nanozymes for practical uses. After that, we also examined the reusability of VS2 as peroxidase catalyst (Fig. S5). Results demonstrate that VS2 is reusable catalyst, which can maintain over 85% of the catalytic activity after even eight repetitive cycles.
Catalytic mechanism of VS2 as POx mimetics
The catalyst-induced generation of reactive oxygen species from the decomposition of H2O2 is often considered to be the key of catalytic activity. To explore the possible mechanism of catalytic behavior of VS2 nanosheets, the catalytic reaction was further investigated in coexistence of different radical scavengers. Here, sodium azide (NaN3), superoxide dismutase (SOD), ascorbic acid (AA) and thiourea were chosen as effective scavengers for 1O2 [23], O2 •− [24], active oxygen free radicals (•OH and O2 •−) [25], and •OH [26], respectively. As exhibited in Fig. 3, the absorbance is not obviously affected in coexistence of NaN3 and SOD, while the absorbance is greatly decreased in coexistence of AA and thiourea. It shows that •OH may be the reactive oxygen species produced in the reaction system. Terephthalic acid can form highly fluorescent product (2-hydroxy terephthalic acid) in the presence of •OH [27]. Therefore, by using terephthalic acid as a fluorescent probe, we further examined the presence of •OH in the catalytic system. The fluorescence emission spectra in Fig. S6 shows that the fluorescence intensity at 425 nm grows with the increase of the VS2 sheets. The above reveals that •OH generates from the VS2-catalyzed decomposition of H2O2 in the reaction system, leading to the oxidation of colorimetric substrate. Therefore, the catalytic mechanism of VS2 peroxidase mimics can be put as follows (Take TMB oxidation as an example):
Further, the apparent steady-state kinetic parameters were determined for the reaction between TMB and H2O2. By plotting the initial reaction velocities against substrate concentrations, typical Michealis-Menten curves were observed for both TMB (Fig. 4a) and H2O2 (Fig. 4b). The curves were then fitted to the Lineweaver Burk plots (Fig. 4c, d), from which the kinetic parameters, Michaelis-Menten constants (K m ) and maximum initial reaction rates (V max ), were calculated.
Generally, a lower K m value represents a higher affinity between the enzyme and the substrate. As showed in Table 1, VS2 nanosheets exhibited lower K m values (0.28 and 3.49 mM) than that of HRP (0.434 and 3.702 mM) [28], indicating higher affinity to both TMB and H2O2. It may originate from the large surface active area and the more reactive sites exposed on the surface of VS2 nanosheets compared to HRP, which has only one iron ion at the active center. In comparison with other POx-like nanozymes in Table 1, VS2 nanosheets also have comparable affinity for both TMB and H2O2. Among them, VS2 catalysts show smaller K m value for TMB than that of their TMDs analogues, such as MoS2 and WS2. Moreover, VS2 exhibit remarkable advantages given by V max over most of the nanozymes in Table 1. Therefore, it is reasonable to claim that VS2 nanosheets exert a superior peroxidase mimicking activity, rivaling natural protein enzyme HRP and many other POx mimetics.
Colorimetric detection assay for H2O2 and glucose
On the basis of the robust peroxidase property of the V2S catalysts, we designed a colorimetric assay for determination of glucose by utilizing the catalyzed TMB–H2O2 colored reaction coupled with glucose oxidase (GOx) (Scheme 1). Briefly, GOx was first incubated with glucose in optimal condition to catalyze the oxidation of glucose to produce gluconic acid and hydrogen peroxide in the presence of oxygen. Subsequently, the formed hydrogen peroxide was utilized to switch on the reaction between the VS2 catalysts and TMB, resulting in the generation of blue color oxidation product. Eventually, the formed color and absorbance was employed as colorimetric signal indirectly probing the glucose content.
To verify the feasibility of this principle, hydrogen peroxide was assigned as detection target directly for the detection assay at first. Figure 5a shows a characteristic absorbance (652 nm) versus H2O2 concentration response plot of the H2O2 assay. The response is linear (R2 = 0.999) in the H2O2 concentration range from 2 to 100 μM with a detection limit (LOD, 3σ) of 0.57 μM (Fig. 5b). This result indicates the VS2-H2O2-TMB reaction system is dependent on H2O2 concentration and suitable for detection assay. Subsequently, glucose detection assay was carried out using VS2-H2O2-TMB reaction combined with glucose-GOx system. Then, the UV-vis absorption intensity at 652 nm was monitored as a function of the glucose concentration. As shown in Fig. 5c, the glucose detection curve is observed to be linear (R2 = 0.996) between 5 and 250 μM with the LOD estimated to be 1.54 μM. Moreover, the VS2 nanosheet-based assay can compete with, or even surpass certain other nanozyme-based glucose assays (see Table S1). The selectivity of the VS2 catalyst for glucose detection was further examined, by monitoring the absorbance change upon addition of various glucose analogues. No significant interference can be observed from fructose, maltose, lactose, or sucrose (Fig. 5d), confirming our colorimetric assay exhibits a good selectivity for glucose. Using this glucose assay, we can detect glucose in food samples such as fruit juices. As shown in Table S2, the results obtained by our colorimetric assay agree with the results obtained by a commercial enzymatic Glucose (HK) Assay Kit (Sigma Aldrich). It validates the reliable colorimetric method for glucose determination in food samples. The good performance of such colorimetric glucose assay based on VS2 nanosheets stimulates promising biocatalytic and analytical applications of VS2 in the future.
H2O2 and glucose detection assays using VS2 POx mimetics. a H2O2 concentration dependent UV-vis absorption change. b H2O2 and (c) glucose assay calibration curves obtained from UV-vis spectra measurement. The inset photograph shows the visually recognizable color change of the reaction system, accordingly. Condition: 50 μg·mL−1 VS2 and 1 mM TMB, 10 min incubation in acetate buffer (pH 4.0) at room temperature. d Selectivity test of glucose over other coexisting substances in fruit juice. The photograph shows the corresponding visual color change. Condition: 1.5 mM glucose and 5 mM other coexisting substances
Conclusion
In conclusion, we have hydrothermally synthesized layered VS2 nanosheets, which are discovered to act as peroxidase-like catalysts to oxidize the peroxidase substrates in the aid of H2O2. Seeing from our results, the VS2 catalysts can be readily available substitute for HRP and many other peroxidase-like nanozymes. It is verified that VS2 nanosheets catalyze the decomposition of H2O2 in acidic pH into •OH, which results in the oxidation of a substrate to form colored reaction product. Relying on this discovery, a colorimetric assay for glucose determination in fruit juice was successfully developed, with high selectivity and low LOD (1.54 μM). In this study, two- dimensional VS2 is demonstrated to be a promising building block for analytical and biological systems. However, to achieve advanced applications, future investigations are still needed in promoting the chemical stability and biocompatibility of VS2. Our work lays the foundation for imitating natural biocatalysis via VS2 nanosheets, facilitating the rise of novel applications in biotechnology and analytical chemistry.
References
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13(4):345–351
Lin Y, Ren J, Qu X (2014) Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res 47(4):1097–1105
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42(14):6060–6093
Huang L, Zhang W, Chen K, Zhu W, Liu X, Wang R, Zhang X, Hu N, Suo Y, Wang J (2017) Facet-selective response of trigger molecule to CeO2 {110} for up-regulating oxidase-like activity. Chem Eng J 330:746–752
Guo Y, Deng L, Li J, Guo S, Wang E, Dong S (2011) Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 5(2):1282–1290
Song Y, Qu K, Zhao C, Ren J, Qu X (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22(19):2206–2210
Vázquez-González M, Liao W-C, Cazelles R, Wang S, Yu X, Gutkin V, Willner I (2017) Mimicking horseradish peroxidase functions using Cu2+-modified carbon nitride nanoparticles or Cu2+-modified carbon dots as heterogeneous catalysts. ACS Nano 11(3):3247–3253
Zhu C, Du D, Lin Y (2017) Graphene-like 2D nanomaterial-based biointerfaces for biosensing applications. Biosens Bioelectron 89:43–55
Zhang W, Shi S, Wang Y, Yu S, Zhu W, Zhang X, Zhang D, Yang B, Wang X, Wang J (2016) Versatile molybdenum disulfide based antibacterial composites for in vitro enhanced sterilization and in vivo focal infection therapy. Nano 8(22):11642–11648
Guo Y, Deng H, Sun X, Li X, Zhao J, Wu J, Chu W, Zhang S, Pan H, Zheng X (2017) Modulation of metal and insulator states in 2D ferromagnetic VS2 by van der Waals interaction engineering. Adv Mater 29(29):1700715-n/a
Yuan J, Wu J, Hardy WJ, Loya P, Lou M, Yang Y, Najmaei S, Jiang M, Qin F, Keyshar K (2015) Facile synthesis of single crystal vanadium disulfide nanosheets by chemical vapor deposition for efficient hydrogen evolution reaction. Adv Mater 27(37):5605–5609
Feng J, Sun X, Wu C, Peng L, Lin C, Hu S, Yang J, Xie Y (2011) Metallic few-layered VS2 ultrathin nanosheets: high two-dimensional conductivity for in-plane supercapacitors. J Am Chem Soc 133(44):17832–17838
Messerschmidt A, Prade L, Wever R (1997) Implications for the catalytic mechanism of the vanadium-containing enzyme chloroperoxidase from the fungus Curvularia inaequalis by X-ray structures of the native and peroxide form. Biol Chem 378(3–4):309–315
Plat H, Krenn BE, Wever R (1987) The bromoperoxidase from the lichen Xanthoria Parietina is a novel vanadium enzyme. Biochem J 248(1):277–279
Robson RL, Eady RR, Richardson TH, Miller RW, Hawkins M, Postgate JR (1986) The alternative nitrogenase of Azotobacter Chroococcum is a vanadium enzyme. Nature 322(6077):388–390
Inzucchi S, Bergenstal R, Fonseca V, Gregg E, Mayer-Davis B, Spollett G, Wender R, Amer Diabet A (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33:S62–S69
Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R (2015) Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov 14(1):45–57
Sanz V, de Marcos S, Castillo JR, Galbán J (2005) Application of molecular absorption properties of horseradish peroxidase for self-indicating enzymatic interactions and analytical methods. J Am Chem Soc 127(3):1038–1048
Qu Y, Shao M, Shao Y, Yang M, Xu J, Kwok CT, Shi X, Lu Z, Pan H (2017) Ultra-high electrocatalytic activity of VS2 nanoflowers for efficient hydrogen evolution reaction. J Mater Chem A 5(29):15080–15086
Zhang W, Wang Y, Zhang D, Yu S, Zhu W, Wang J, Zheng F, Wang S, Wang J (2015) A one-step approach to the large-scale synthesis of functionalized MoS2 nanosheets by ionic liquid assisted grinding. Nano 7(22):10210–10217
Yin W, Yu J, Lv F, Yan L, Zheng LR, Gu Z, Zhao Y (2016) Functionalized nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano 10(12):11000–11011
Shuai H-L, Huang K-J, Chen Y-X (2016) A layered tungsten disulfide/acetylene black composite based DNA biosensing platform coupled with hybridization chain reaction for signal amplification. J Mater Chem B 4(6):1186–1196
Harbour JR, Issler SL (1982) Involvement of the azide radical in the quenching of singlet oxygen by azide anion in water. J Am Cheml Soc 104(3):903–905
Schaap AP, Thayer AL, Faler GR, Goda K, Kimura T (1974) Singlet molecular oxygen and superoxide dismutase. J Am Chem Soc 96(12):4025–4026
Su L, Feng J, Zhou X, Ren C, Li H, Chen X (2012) Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal Chem 84(13):5753–5758
Wang W-F, Schuchmann MN, Schuchmann H-P, Knolle W, von Sonntag J, von Sonntag C (1999) Radical cations in the OH-radical-induced oxidation of thiourea and tetramethylthiourea in aqueous solution. J Am Chem Soc 121(1):238–245
Dalui A, Pradhan B, Thupakula U, Khan AH, Kumar GS, Ghosh T, Satpati B, Acharya S (2015) Insight into the mechanism revealing the peroxidase mimetic catalytic activity of quaternary CuZnFeS nanocrystals: colorimetric biosensing of hydrogen peroxide and glucose. Nano 7(19):9062–9074
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Lin T, Zhong L, Song Z, Guo L, Wu H, Guo Q, Chen Y, Fu F, Chen G (2014) Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens Bioelectron 62:302–307
Lin T, Zhong L, Guo L, Fu F, Chen G (2014) Seeing diabetes: visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nano 6(20):11856–11862
Ding C, Yan Y, Xiang D, Zhang C, Xian Y (2016) Magnetic Fe3S4 nanoparticles with peroxidase-like activity, and their use in a photometric enzymatic glucose assay. Microchim Acta 183(2):625–631
Cai R, Yang D, Peng S, Chen X, Huang Y, Liu Y, Hou W, Yang S, Liu Z, Tan W (2015) Single nanoparticle to 3D Supercage: framing for an artificial enzyme system. J Am Chem Soc 137(43):13957–13963
Wang N, Sun J, Chen L, Fan H, Ai S (2015) A Cu2(OH)3Cl-CeO2 nanocomposite with peroxidase-like activity, and its application to the determination of hydrogen peroxide, glucose and cholesterol. Microchim Acta 182(9):1733–1738
Wang B, Ju P, Zhang D, Han X, Zheng L, Yin X, Sun C (2016) Colorimetric detection of H2O2 using flower-like Fe2(MoO4)3 microparticles as a peroxidase mimic. Microchim Acta 183(11):3025–3033
Zhong Y, Deng C, He Y, Ge Y, Song G (2016) Exploring a monothiolated β-cyclodextrin as the template to synthesize copper nanoclusters with exceptionally increased peroxidase-like activity. Microchim Acta 183(10):2823–2830
Acknowledgements
This research was financed by Grants from National Natural Science Foundation of China (No. 21675127), Fundamental Research Funds for the Northwest A&F University of China (2014YB093, 2452015257), and Development Project of Qinghai Key Laboratory (No. 2017-ZJ-Y10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2000 kb)
Rights and permissions
About this article
Cite this article
Huang, L., Zhu, W., Zhang, W. et al. Layered vanadium(IV) disulfide nanosheets as a peroxidase-like nanozyme for colorimetric detection of glucose. Microchim Acta 185, 7 (2018). https://doi.org/10.1007/s00604-017-2552-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2552-1