Abstract
The article describes a magnetic metal-organic framework (MOF) of the type MIL-101(Fe)/2-(propylamino-ethyl) dithiocarbamate on the surface of magnetite nanoparticles. The MOF is shown to be a viable material for speciation analysis of Cr(III) and Cr(VI) because it shows selectivity for Cr(VI) at pH values around 2.0, while at pH values around 5 both Cr(III) and Cr(VI) species are sorbed. Hence, preoxidation or reduction treatments are not needed. After optimization of the extraction conditions, chromium was quantified by ET-AAS. Feature of the determination of Cr(VI) include (a) a 1.0 ng L−1 limit of detection, (b) a linear analytical range that extends from 3 to 300 ng L−1, and (c) a relative standard deviation of 6.4%. The respective values for total chromium are 1.5 ng L−1, 4 to 325 ng L−1 and 7.5%, respectively. The method was validated by analyzing two certified reference materials. It also was successfully employed to the rapid extraction and speciation of Cr(III) and Cr(VI) in (spiked) water samples and of total chromium in tea samples.

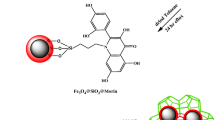
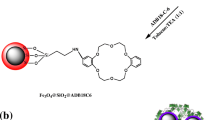
A novel magnetic metal-organic framework [a MIL-101(Fe)/2-(propylamino-ethyl) dithiocarbamate modified magnetite nanoparticle composite] was synthesized and utilized for speciation analysis of Cr(III) and Cr(VI) via determination by electrothermal atomic absorption spectrometry. (a) A schematic representation for synthesis of Fe3O4@PAEDTC NPs. (b) Schematic illustration of the synthesis of MIL-101(Fe)/Fe3O4@ PAEDTC nanocomposite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal-organic frameworks (MOFs) or porous coordination polymers are a new type of porous crystalline materials which assembled via bonding of organic bridging ligands with metal centers [1]. Owing to their unique structural diversity and excellent properties, MOFs have attracted considerable attention in the analytical chemistry and have been early investigated as excellent sorbents for the analysis of various environmental pollutants such as polybrominated diphenyl ethers [1], pesticides [2], polycyclic aromatic hydrocarbons [3], parabens [4], plasticizers [5] and heavy metal ions [6–10]. MOFs can be employed as ideal sorbents owing to their unique properties such as very high surface areas, flexible and highly porous structure and great diversity in surface functionalization [11]. These unique properties make the MOFs excellent sorbents in extraction and preconcentration of various analytes.
Environmental pollution character of heavy metals and metalloids has attracted great attention owing to their high toxicity. Heavy metals have a high tendency to form complexes with various compounds, particularly biological ligands containing nitrogen, sulfur, and oxygen atoms [12]. The complex formation can resulted to changes in the molecular conformation of proteins, enzymes inhibition and hydrogen bonds breaking. One of these heavy metals is chromium. The toxicity of chromium is related to its oxidation states. Two dominant oxidation states of chromium in the environment are Cr(III) and Cr(VI) [13]. These two species have very different properties. In one hand, Cr(III) is considered as an essential element in organisms [14, 15] for the maintenance of glucose, lipid and protein metabolism [16]. The role of insulin hormone, which controls the blood sugar levels, is depends to Cr(III) [17]. Besides, Cr(III) compounds are slightly soluble except in cases where complex formation is occurred [18]. On the other hand, Cr(VI) species such as CrO4 2−, HCrO4 2− and Cr2O7 2− are highly toxic and hazardous to human and other mammals due to their adverse effect on various organisms such as lung, liver and kidney. These species are categorized as carcinogenic agents even at very low levels [19, 20]. Moreover, Cr(VI) ions are very mobile and soluble in water samples and are not adsorbed into many soils [15, 21]. As chromium contamination in natural waters is considered a global concern, the World Health Organization (WHO) guideline value set for Cr(VI) in drinking water is 50 μg L−1 for daily intake. However, this value is very high compared to the high risk of carcinogenicity of Cr(VI). Hence, Cr speciation in real sample is critical since the quantification of total chromium in diverse sample matrixes is no longer sufficient to indicate the risks of chromium to the environment and also human health [22, 23]. Thereby, monitoring and quantification of very low levels of Cr(III) and Cr(VI) is often a major task for the analytical chemists, as it is a good tool for the identification, monitoring and determination of this toxic species in environmental samples. Hence, development easy, quick, sensitive and environmentally friendly methods for Cr(III) and Cr(VI) monitoring and quantification are of particular significance.
The concentration of chromium species in real samples is often very low and most of these samples have complicated matrices; thereby a separation and preconcentration step is necessary for precise and accurate quantification [24]. Hence, various preconcentration and extraction methods such as solvent extraction [25, 26], co-precipitation [27], cloud point extraction [22, 23] and solid phase extraction (SPE) [21, 28, 29] have been employed for preconcentration and speciation analysis of Cr(III) and Cr(VI) ions. Owing to its simplicity, rapidity, low consumption of reagents and minimal cost, SPE is the most universally employed extraction method for preconcentration of metal species in different environmental and food samples [30]. However, in most cases the fast and complete isolation, and removal of sorbents from extraction media is difficult, time-consuming, and labor-intensive which can cause to additional environmental problems [31]. Employing a magnetic sorbent facilitates the separation of solid material from solution, by employing an external magnetic field, and accelerates sample preparation procedures [10, 31].
The aim of this work is extraction of Cr(III) and Cr(VI) species by means of a novel magnetic metal organic framework and their determination by electrothermal atomic absorption spectrometry (ETAAS). Initially, Fe3O4@SiO2@2-(propylamino-ethyl) dithiocarbamate nanoparticles (Fe3O4@PAEDTC NPs) were synthesized and then immobilized in the structure of metal-organic framework (MIL-101(Fe)). The nanocomposite was characterized by Fourier transform infrared spectroscopy (FT-IR), BET analysis, scanning electron microscopy (SEM), CHNS analysis and vibrating sample magnetometry (VSM). The magnetic characteristic of nanosorbent simplified and accelerated the separation and recovery of the sorbent from extraction medium. The presence of amine and dithiocarbamate moieties in the structure of nanosorbent led to its selectivity towards chromium species. Design of experiments approach through response surface methodology was employed in order to find the optimum operating conditions of the extraction method. Finally, the magnetic solid phase extraction (MSPE) method was used for determination of Cr(III) and Cr(VI) species in water and tea samples.
Experimental
Reagents and instrumentation
All details about reagents and instrumentation are mentioned in Electronic Supplementary Information.
Preparation of standard solutions
Stock solutions (1000 mg L−1) of potentially interfering ions such as K+, Na+, Mg(II), Ca(II), Mn(II), Pb(II), As(III), As(V), Al(III), Cd(II), Cu(II), Zn(II), Co(II), Cl− and NO3 − were prepared in a 2% (v/v) HNO3 solution. The working standard solutions were prepared by diluting an appropriate amount of the stock solution with double distilled water. All of these solutions were stored at ambient temperature prior to use.
Synthesis of sorbents
Synthesis of 2-(propylamino-ethyl) dithiocarbamate functionalized Fe3O4 NPs
Fe3O4 NPs were synthesized according to a previously reported procedure [10]. Briefly, exact amount of FeCl3 (7.2 g) and (NH4)2Fe(SO4)2.6H2O (7.84 g) salts were dissolved in 400 mL deionized water and degassed with N2 for 10 min and then heated to 80 °C. Thereafter, 20 mL ammonia solution (25% w/w) was added to the reaction mixture under nitrogen gas protection and vigorous stirring (1000 rpm). During the whole process, the temperature was maintained at 80 °C and nitrogen gas was used to prevent the penetration of oxygen. After the reaction, magnetite NPs was separated from the reaction medium by the magnetic decantation, and then washed ten times with 200 mL deionized water until the pH became neutral. The obtained MNPs were dried in room temperature. In the next step, 1.0 g of prepared Fe3O4 NPs was suspended in a solution of 250 mL deionized water, 75 mL ethanol and 4 mL NH4OH (28%). Afterwards, 3.0 mL TEOS was added slowly to the mixture under vigorous stirring (1000 rpm) [32]. After 10 h stirring at 40 °C, Fe3O4@SiO2 NPs were gathered by a strong magnet (15 cm × 12 cm × 5 cm, 1.4 T), washed with ethanol (3 × 50 mL) and finally dried at room temperature [33]. In the next step, 1.0 g of Fe3O4@SiO2 NPs was suspended in 100 mL dried toluene, and the mixture was stirred for 60 min. Thereafter, 1.0 mL AEAPTMS was added to the reaction mixture and it was refluxed for 15 h and then the solid was removed from the solvents by magnetic separation and washed with methanol (3 × 25 mL) and (2 × 40 mL) acetone and then dried at room temperature (Fig. 1a). In order to prepare PAEDTC-functionalized NPs, 1.0 g dried Fe3O4@SiO2@EN NPs was dispersed in 150 mL 2-propanol for 60 min. Thereafter, 3 mL NaOH 1 mol L−1 and 2.4 mL CS2 were added to the reaction mixture and the solution was stirred at room temperature for 4 h (Fig. 1a). Finally, Fe3O4@SiO2@PAEDTC NPs was dried at 50 °C after washing with water (5 × 50 mL) and methanol (3 × 25 mL). The synthesis of Fe3O4@SiO2@PAEDTC was investigated by FT-IR spectroscopy, TEM, SEM and VSM techniques.
Synthesis of MIL-101/dithiocarbamate magnetite nanoparticles composite
MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite was prepared according to the following procedure (Fig. 1b). Briefly, 1.03 g H2BDC was dissolved in 50 mL of DMF and sonicated for 10 min (mixture A). Then, 0.5 g Fe3O4@PAEDTC NPs was suspended in a solution containing 40 mL DMF and 3.38 g FeCl3.6H2O (mixture B). In the next step, mixture A and B were transferred into an autoclave and it was heated at 110 °C for 24 h. Finally, MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite was recovered from the supernatant solution by magnetic decantation and washed with water (5 × 25 mL) and ethanol (3 × 50 mL), respectively and dried at room temperature. The nanocomposite was characterized by FT-IR, VSM, CHNS analysis, SEM and BET analysis.
Synthesis of MIL-101(Fe)
MIL-101(Fe) was prepared according to the previously reported procedure [34]. A total amount of 1.03 g of H2BDC and 3.25 g of FeCl3.6H2O was mixed with 100 mL of DMF for 15 min under sonication. The solution was transferred into an autoclave and was heated at 110 °C for 24 h. Finally, the powder was separated by centrifugation, washed once (100 mL) with water and then with ethanol four times (4 × 20 mL) to remove unreacted reagents and was dried under vacuum at 100 °C for 16 h.
Extraction procedure
Extraction was conducted in test tubes containing 30 mL 0.1 μg L−1 solution of Cr(III) and Cr(VI). This solution divided into two equal 15.0 mL, which are named as sample 1 and 2. Thereafter, pH of sample 1 was adjusted to 2.0 using 0.1 mol L−1 HCl solution. Afterwards, 14.8 mg of magnetic nanocomposite was added to the mixture and was stirred for 6.5 min in order to extract Cr(VI) ions from the solution, completely. Then the sorbent was separated from the solution by using the strong magnet. The sorbed amount of Cr(VI) ions was determined using ETAAS based on its concentration change after the sorption step. In the next step, 0.85 mL of 0.6 mol L-l EDTA in 0.3 mol L-l HNO3 solution as an eluent was added to the test tube containing the sorbent and it was stirred for 12.2 min. Finally, the mixture was again exposed to the strong magnet to separate the sorbent from the media. The clear solution containing eluted Cr(VI) ions was injected to ETAAS for subsequent analysis. The same opration conditions was employed for the extraction and determination of total Cr from solution 2 except the pH of sample which was adjusted to 5.0. The concentration of Cr(III) in the sample was obtained by subtracing Cr(VI) concentration (sample 1) from the total Cr concentration (sample 2).
Real sample pretreatment
All details about real sample oretreatment are mentioned in Electronic Supplementary Information.
Results and discussion
Choice of materials
The sorbent type actually is a vital variable affecting the extraction efficiency. Owing to the high surface area of MIL-101 (2310 m2 g−1) and the excellent magnetic properties of magnetite NPs, magnetic MIL-101 (Fe) was used as a starting material for sorption of Cr species. Magnetite NPs were covered with a silica layer in order to improve their chemical stability in acidic medium. In the case of metal ions extraction, commonly, solutions of strong acids are used as eluent which may dissolve Fe3O4 NPs, so a protecting layer such as SiO2 should be used. Moreover, silica surface is often terminated by silanol groups that can react with silane coupling agents in order to conjugate with a variety of specific ligands. After synthesis of nanosorbent, extraction capabilities of bare MIL-101(Fe), Fe3O4@PAEDTC NPs and MIL-101(Fe)/ Fe3O4@PAEDTC nanocomposite were studied. As indicated in Fig. 1S (Electronic Supplementary Data) MIL-101(Fe)/Fe3O4@PAEDTC nanosorbent shows the highest extraction efficiency among different sorbents owing to the high surface area and presence of S and N atoms in its structure. The existence of Fe3O4@PAEDTC NPs on the surface MIL-101(Fe) and Fe3O4 NPs enhances the complex formation between the target analytes and hetro atoms of the sorbent while MIL-101(Fe) acts as a support and spacer and prevents the aggregation of Fe3O4@PAEDTC NPs [31]. In comparison with Fe3O4@PAEDTC NPs, MIL-101(Fe)/Fe3O4@PAEDTC composite offer a significantly higher surface area to volume ratio due to the presence of MIL-101(Fe). Thereby, MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite was selected as the most appropriate sorbent for the rest of the studies.
Characterization
FT-IR spectroscopy and CHNS analysis
The FT-IR spectrum of MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite was obtained by employing KBr pellet method. The presence of the absorption peaks related to C-H aliphatic (2950 and 2893 cm−1), C = C (1464 cm−1), C-N (1441 cm−1), C = S (1230 cm−1), N-H (790 cm−1), Fe-O (583 cm−1) and Si-O-Si (1042 cm−1) confirmed the functionalization of MIL-101(Fe) with Fe3O4@PAEDTC NPs. The CHNS analysis was conducted to study the elemental contents of MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite. The results demonstrate 34.4% C, 1.5% H, 2.4% N and 3.6% S in the structure of the magnetic nanocomposite which indicate that Fe3O4@PAEDTC NPs have been sufficiently immobilized in the structure of MIL-101(Fe).
SEM and TEM characterization
To investigate the surface morphology of Fe3O4@PAEDTC NPs and MIL-101(Fe)/ Fe3O4@PAEDTC nanocomposite, the samples were characterized by TEM (Fig. 2) or SEM (Fig. 2S, Electronic Supplementary Data). The crystals of MIL-101(Fe) sample have a smooth surface and their average size was 400 nm (Fig. 2S) while the surface of MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite was rough after modification with Fe3O4@PAEDTC NPs (Fig. 2S) and revealed the successful immobilization of Fe3O4@PAEDTC on the surface of MIL-101(Fe). The TEM image of Fe3O4@PAEDTC NPs demonstrated a core-shell structure (Fig. 2) with an electron dense region which is related to nanosized Fe3O4 cores (20–30 nm) and a less dense and more transparent layer around Fe3O4 cores. This outer layer is corresponds to SiO2 and dithiocarbamate coating shell (10–15 nm).
VSM and BET analyses
All details about VSM and BET analyses are mentioned in Electronic Supplementary Information.
Optimization study
The following parameters were optimized: (a) sample pH value; (b) sorption step; (c) the eluent; (c) the elution step. Respective data and Figures are given in the Electronic Supporting Material (Fig. 1S, 3S, 4S and 5S, Table 2S). The following experimental conditions were found to give best results: sample pH values of 2.0 for Cr(VI) and 5.0 for total Cr sorption; a sorption time of 6.5 min; and the use of 14.8 mg of the magnetic nanosorbent in the sorption step, and the use of a 0.3 mol L-l HNO3 in 0.6 mol L-l EDTA solution as eluent; an eluent volume of 0.85 mL; and an elution time of 12.2 min.
Effect of the potentially interfering ions
The effect of some potentially interfering ions such as K+, Na+, Mg(II), Ca(II), Mn(II), Pb(II), As(III), As(V), Al(III), Cd(II), Cu(II), Zn(II), Co(II), Cl− and NO3 − on extraction efficiency of Cr(III) and Cr(VI) was investigated by addition of each mentioned ion to 100 mL of a solution containing 0.1 μg Cr(III) and Cr(VI) ions. The extraction recoveries of Cr(III) and Cr(VI) in the individual binary mixture solutions are presented in Table 3S (Electronic Supplementary Data). Based the results, it can be concluded that even high concentration of the potentially interfering ions did not influence the extraction recovery of Cr ions, hence selective extraction of Cr(III) and Cr(VI) in the case of real sample can be achieved.
Effect of sample volume and sorption capacity study
In the case of real samples, the sample volume is one of the very important factors influencing the preconcentration factor. Thereby, the effect of sample volume was studied by dissolving 0.01 mg of Cr(III) and Cr(VI) ions in 100, 200, 300, 400, 500 and 600 mL distilled water. Afterwards, the method was conducted and the results exhibit that Cr ions can be recovered for sample volumes of 400 mL. Hence, a preconcentration factor of 470 was achieved for Cr ions extraction using MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite.
The sorption capacity of MIL-101(Fe)/Fe3O4@PAEDTC sorbent was studied using a standard solution containing 1 mg L−1 of Cr(III) and Cr(VI) ions under the opted conditions at pH = 5.0. Briefly, the equilibrium concentration of total chromium after sorption process was determined by ETAAS. The maximum sorption capacity is defined as the total amount of Cr(III) and Cr(VI) sorbed ions (mg) per gram of the nanosorbent which was 335 mg g−1 and demonstrated the high capacity of the sorbent owing to its porous and nano-sized structure.
Analytical figures of merit of the method
Under the opted operating conditions, linearity was obtained within the range of 3–300 ng L−1 for Cr(VI) and 4–325 ng L−1 for total Cr in initial solution with correlation of determination (r2) equal to 0.9931 and 0.9945, respectively. The limit of detection was calculated as LOD = 3Sb/m, where Sb is the standard deviation of 7 replicate blank signals and m is the slope of the calibration curve after extraction process. LOD was found to be 1.0 ng L−1 for Cr(VI) and 1.5 ng L−1 for total Cr in a sample volume of 400 mL. The precision of the method for a standard solution containing 30 ng L−1 of Cr(III) and Cr(VI) ions (n = 5) was evaluated as the relative standard deviation (RSD%) and was found to be 6.4% for Cr(VI) and 7.5% for total Cr. Moreover, the analytical features of MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite and bare MIL-101(Fe) was compared (Table 4S). As it is tabulated in this paper, MIL-101(Fe)/Fe3O4@PAEDTC nanocomposite has a higher sorption capacity and extraction recovery and lower detection limit toward Cr(VI) compared which can be attributed to the presence of Fe3O4@PAEDTC NPs in the structure of nanocomposite.
Validation of the method
The accuracy of method was evaluated by using two certified reference materials (SRM 1640 natural water, GBW 07605 tea). For this purpose, the concentration of total chromium was determined under the optimized operating conditions. As shown in Table 1, the results are in good agreement with the real amount of chromium in two SRM. Hence, the extraction method can be utilized as a reliable method for the preconcentration and determination of Cr in various samples.
Determination of Cr(III) and Cr(VI) and total chromium in real samples
The applicability of the extraction method was investigated by analyzing water and tea samples under the optimized operating conditions in order to reduce undesirable matrix effect. It is necessary to note that in the case of water samples Cr(III) and Cr(VI) was determined, while in the analysis of black and green tea samples total amount of chomium was quantified. Table 2 depicts the chromium recoveries in various samples which in all cases, were almost quantitative (86.0–110%) and are in good agreement with spiked levels.
Conclusion
In this study, a novel MIL-101(Fe)/Fe3O4@2-(propylamino-ethyl) dithiocarbamate nanocomposite was employed as a viable sorbent for preconcentration, extraction and speciation analysis of Cr(III) and Cr(VI) ions. The method was fast, simple, selective, accurate and precise and did not need any oxidation or reduction pretreatments. Immobilization of Fe3O4@Fe3O4@2-(propylamino-ethyl) nanoparticles at MIL-101(Fe) surface led to it selectivity towards Cr(VI) at pH = 2.0 and total chromium (Cr(III) and Cr(VI) ions) at pH = 5.0. This new magnetic solid phase extraction exhibited the advantages of high sorption capacity (335 mg g−1), high enrichment factor (470 times), fast extraction time (20 min) and low detection limit (1.0–1.5 ng L−1) compared to previously reported methods (Table 3). The sorption capacity of the nanocomposite was higher than that of bare MIL-101(Fe) and Fe3O4@2-(propylamino-ethyl) dithiocarbamate NPs. Hence, the analytical performance of the method is desirable. The method was validated using two certified reference materials (SRM 1640 natural water and GBW 07605 tea) to confirm its accuracy. Ultimately, the method in combination with ETAAS was employed for the fast extraction and speciation of ultra trace chromium in water and tea samples, satisfactorily. Besides, this sorbent can be employed for the speciation analysis of other metal ions. The complicated process of sorbent preparation is a limitation of this work. However, large amounts of the sorbent can be synthesis in one batch only.
References
Zhang CY, Yan ZG, Zhou YY, Wang L, Xie YB, Bai LP, Zhou HY, Li FS (2015) Embedment of Ag (I)-organic frameworks into silica gels for microextraction of polybrominated diphenyl ethers in soils. J Chromatogr A 1383:18–24
Zhang S, Jiao Z, Yao W (2014) A simple solvothermal process for fabrication of a metal-organic framework with an iron oxide enclosure for the determination of organophosphorus pesticides in biological samples. J Chromatogr A 1371:74–81
Huo SH, Yan XP (2012) Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples. Analyst 137:3445–3451
Rocío-Bautista P, Martínez-Benito C, Pino V, Pasán J, Ayala JH, Ruiz-Pérez C, Afonso AM (2015) The metal-organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine. Talanta 139:13–20
Tahmasebi E, Masoomi MY, Yamini Y, Morsali A (2016) Application of a Zn(II) based metal-organic framework as an efficient solid-phase extraction sorbent for preconcentration of plasticizer compounds. RSC Adv 6:40211–40218
Babazadeh M, Hosseinzadeh-Khanmiri R, Abolhasani J, Ghorbani-Kalhor E, Hassanpour A (2015) Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal-organic framework. RSC Adv 5:19884–19892
Ghorbani-Kalhor E, Hosseinzadeh-Khanmiri R, Babazadeh M, Abolhasani J, Hassanpour A (2015) Synthesis and application of a novel magnetic metal-organic framework nanocomposite for determination of Cd, Pb, and Zn in baby food samples. Can J Chem 93:518–525
Sohrabi MR, Matbouie Z, Asgharinezhad AA, Dehghani A (2013) Solid phase extraction of Cd (II) and Pb (II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim Acta 180:589–597
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Tadjarodi A, Abbaszadeh A (2016) A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury(II). Microchim Acta 183:1391–1399
Wang Y, Chen H, Tang J, Ye G, Ge H, Hu X (2015) Preparation of magnetic metal organic frameworks adsorbent modified with mercapto groups for the extraction and analysis of lead in food samples by flame atomic absorption spectrometry. Food Chem 181:191–197
Bagheri H, Afkhami A, Saber-Tehrani M, Khoshsafar H (2012) Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 97:87–95
Gode F, Pehlivan E (2005) Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. J Hazard Mater 119:175–182
El-Sheikh AH, Al-Degs YS, Sweileh JA, Said AJ (2013) Separation and flame atomic absorption spectrometric determination of total chromium and chromium(III) in phosphate rock used for production of fertilizer. Talanta 116:482–487
Rezvani M, Asgharinezhad AA, Ebrahimzadeh H, Shekari N (2014) A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium(VI) ions. Microchim Acta 181:1887–1895
Noroozifar M, Khorasani-Motlagh M, Gorgij MN, Naderpour HR (2008) Adsorption behavior of Cr(VI) on modified natural zeolite by a new bolaform N, N, N, N′, N′, N′-hexamethyl-1,9-nonanediammonium dibromide reagent. J Hazard Mater 155:566–571
Nielsen S, Hansen EH (1998) Selective flow-injection quantification of ultra-trace amounts of Cr(VI) via on-line complexation and preconcentration with APDC followed by determination by electrothermal atomic absorption spectrometry. Anal Chim Acta 366:163–176
Liang P, Ding Q, Liu Y (2006) Speciation of chromium by selective separation and preconcentration of Cr(III) on an immobilized nanometer titanium dioxide microcolumn. J Sep Sci 29:242–247
Uluozlu OD, Tuzen M, Soylak M (2009) Speciation and separation of Cr(VI) and Cr(III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem Toxicol 47:2601–2605
Ebrahimzadeh H, Asgharinezhad AA, Tavassoli N, Sadeghi O, Amini MM, Kamarei F (2012) Separation and spectrophotometric determination of very low levels of Cr(VI) in water samples by novel pyridine-functionalized mesoporous silica. Int J Environ Anal Chem 92:509–521
Islam A, Ahmad H, Zaidi N, Kumar S (2016) A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim Acta 183:289–296
Kiran K, Kumar KS, Prasad B, Suvardhan K, Lekkala RB, Janardhanam K (2008) Speciation determination of chromium (III) and (VI) using preconcentration cloud point extraction with flame atomic absorption spectrometry (FAAS). J Hazard Mater 150:582–586
Ezoddin M, Shemirani F, Khani R (2010) Application of mixed-micelle cloud point extraction for speciation analysis of chromium in water samples by electrothermal atomic absorption spectrometry. Desalination 262:183–187
Saxena R, Tiwari S, Sharma N (2015) Flow-injection solid phase extraction using Dowex Optipore L493 loaded with dithizone for preconcentration of chromium species from industrial waters and determination by FAAS. RSC Adv 5:69196–69204
Sadeghi S, Moghaddam AZ (2012) Preconcentration and speciation of trace amounts of chromium in saline samples using temperature-controlled microextraction based on ionic liquid as extraction solvent and determination by electrothermal atomic absorption spectrometry. Talanta 99:758–766
Sadeghi S, Moghaddam AZ (2016) Multiple response optimization of sequential speciation of chromium in water samples by in situ solvent formation dispersive liquid–liquid microextraction prior to electrothermal atomic absorption spectrometry determination. J Iran Chem Soc 13:117–130
Duran A, Tuzen M, Soylak M (2011) Speciation of Cr(III) and Cr(VI) in geological and water samples by ytterbium(III) hydroxide coprecipitation system and atomic absorption spectrometry. Food Chem Toxicol 49:1633–1637
Tuzen M, Soylak M (2007) Multiwalled carbon nanotubes for speciation of chromium in environmental samples. J Hazard Mater 147:219–225
Sadeghi S, Moghaddam AZ (2014) Solid-phase extraction and HPLC-UV detection of Cr(III) and Cr(VI) using ionic liquid-functionalized silica as a hydrophobic sorbent. Anal Methods 6:4867–4877
Habila MA, ALOthman, ZA, El-Toni, AM, Labis, JP, Li, X, Zhang, F, Soylak, M (2016) Mercaptobenzothiazole-functionalized magnetic carbon nanospheres of type Fe3O4@SiO2@C for the preconcentration of nickel, copper and lead prior to their determination by ICP-MS. Microchim Acta 1–8
Asgharinezhad AA, Ebrahimzadeh H (2016) Poly (2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J Chromatogr A 1435:18–29
Sadeghi S, Aboobakri E (2012) Magnetic nanoparticles with an imprinted polymer coating for the selective extraction of uranyl ions. Microchim Acta 178:89–97
Abolhasani J, Khanmiri RH, Ghorbani-Kalhor E, Hassanpour A, Asgharinezhad AA, Shekari N, Fathi A (2015) An Fe3O4@SiO2@polypyrrole magnetic nanocomposite for the extraction and preconcentration of Cd(II) and Ni(II). Anal Methods 7:313–320
Bauer S, Serre C, Devic T, Horcajada P, Marrot J, Férey G, Stock N (2008) High-throughput assisted rationalization of the formation of metal organic frameworks in the iron (III) aminoterephthalate solvothermal system. Inorg Chem 47:7568–7576
Abdolmohammad-Zadeh H, Sadeghi GH (2012) A nano-structured material for reliable speciation of chromium and manganese in drinking waters, surface waters and industrial wastewater effluents. Talanta 94:201–208
Yao XP, Fu ZJ, Zhao YG, Wang L, Fang LY, Shen HY (2012) Use of tetraethylenepentamine-functional Fe3O4 magnetic polymers for matrix solid phase dispersion extraction and preconcentration of Cr(VI) in water samples at ultratrace levels. Talanta 97:124–130
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declare that he has no competing interests.
Electronic supplementary material
ESM 1
(DOCX 931 kb)
Rights and permissions
About this article
Cite this article
Saboori, A. A nanoparticle sorbent composed of MIL-101(Fe) and dithiocarbamate-modified magnetite nanoparticles for speciation of Cr(III) and Cr(VI) prior to their determination by electrothermal AAS. Microchim Acta 184, 1509–1516 (2017). https://doi.org/10.1007/s00604-017-2155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2155-x