Abstract
The paper reports on a method for the detection of nanocellulose (NC) in consumer products by making use of a combination of (a) liquid-liquid extraction with an ionic liquid, and (b) size characterization by asymmetric flow field-flow fractionation (AF4) coupled to multi-angle light scattering (MALS) and refractive index (RI) detection. Both AF4 and MALS are viable tools for characterizing the size of the nanofibers. Sample preparation is easy, and the extraction efficiency of the method is 80.9 ± 1.8% (n = 5). It was applied to the detection of NC in toothpaste and coconut foodstuff to verify the practicability of the method.

Nanocellulose (NC) monitoring from coconut products and toothpaste and its size characterization by liquid-liquid extraction and asymmetric flow field-flow fractionation equipped with multi-angle light scattering and refractive index detection (AF4-MALS-RI).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials encountered in food can be of natural origin (i.e., coming from the ingredients), or can be introduced as a result of advanced food packaging with the aim to improve shelf life [1]. Engineered nanoparticles (NPs) are often added as additives or supplements to consumer products to preserve and enhance taste, appearance and aroma. However, most of the consumer products are unlabeled or actually inappropriate labeled by avoiding the term “nano”, without specifying the amount/ type of the nanomaterial used or even without indicating the origin from which the NPs were supplied.
Due to the increase number of toxicological studies of engineered NPs and the growing consumption of consumer products containing NPs, the European Union called fast and cost-effective methods to control and monitor them; it is important to know the type of nanomaterials and the doses incorporated into the consumer products for the safety of consumers. The problem facing researchers is related to the analyses of small quantities of a large variety of complex matrices containing NPs both quickly and at a reasonable cost.
Characterization of engineered NPs is an area of great interest, but limited methods for their extraction, detection and quantification are established. The goal of this paper is to achieve a general method to extend their applicability to a wide range of samples.
Nanocellulose (NC) has garnered unprecedented interest in the last decade for their potential in cosmetics and in food industry [2] as a result of the appealing intrinsic properties: renewability, biodegradability, large surface area, low density, high strength and stiffness, chiral ordering and cylindrical morphology in the nanoscale, amongst others. In cosmetic and food technology, NC displays moisturizing and stabilizing properties and acts as thickener [3]; it is also excellent filler in bioactive packaging materials for efficiently recycling by virtue of its strong reinforcement effect and reducing of damage of products by wear, UV rays and humidity [4]. Concerning the preparation of NC, nature is able to assemble cellulose polymers to form highly ordered nanostructures by virtue of the hydrogen bonding; however, the humanity takes advantage of top-down methodologies to isolate NC from natural resources like micro- and macrofiber bundles from plants and trees, being their isolation extensively described [5–9]. Different green alternatives regarding their isolation from cotton, plants and wastes were reported in the last decade [10]. At first, the multifaceted advantages of NC and its low-toxicity make this NP widely used as dietary fibers, but it is crucial to understand the lack of toxicological information related to the exposure limits and side effects [11]. Taking into account the role of NC in commercially available food and cosmetics (as means to produce foams, emulsions and gels [12]), alternative methods to monitor, identify and quantify NC are required; thus, monitoring such NPs the upcoming legislation can fulfill the regulatory gaps related to the nanosafety. Chromatographic instrumentations as well as RAMAN and fluorescence spectroscopies are subjected to a range of limitations for NC analysis; however, field-flow fractionation [13] seems to be a very powerful technique for NP characterization [14–17] and monitoring [18–22].
Asymmetric flow field-flow fractionation (AF4) [23, 24] is a non-destructive chromatographic technique useful for the rapid separation of the particle accordingly to differences in particle mobility along a trapezoidal channel. The separation is produced through a parabolic flow profile divided into two components: a longitudinal channel flow consisted on different velocity streamlines and, a transversal cross flow which promoted the movement of particles through the accumulation wall. Under those conditions, NPs coexisted in a specific high, and thus, speed accordingly to their size and diffusion coefficient. The final velocity gradient produced by both flows inside the channel enables to separate the particles by their size [25]. According to the AF4 theory, the smaller particles are diffused faster across the channel, and so eluted earlier than the larger ones (Fig. S1).
To the best of our knowledge, no description of the extraction of cellulose nanofibrils from commercial products as complex matrices were reported. The aim of this work is to extract and characterize NC in different consumer products (food [3] and deodorant [26]). On the one hand, the challenge of a general protocol for the extraction and preconcentration of NC from food and cosmetics is addressed; this involves a simple and fast two-step method based on the use of ionic liquid for extracting NC in presence of a cationic surfactant. On the other hand, a versatile detection method for the separation and characterization accordingly to their size is presented; thus, the extracted NC from different matrices were analyzed by AF4 with multi-angle light scattering detector (MALS). Dynamic light scattering (DLS) was used to confirm the AF4-MALS results, and Fourier transform infrared spectroscopy (FT-IR) spectroscopy to assure the integrity of NC after the extraction procedure.
Experimental
Reagents and materials
Avicel PH-101 microcrystalline cellulose (MCC, 50 μm of particle size), 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO, 98%), sodium hypochlorite solution (NaClO, 10–15%), sodium bromide (>99%), ethylenediaminetetraacetic acid (EDTA, 99%), hexadecyltrimethylammonium chloride and bromide (CTAC, CTAB, >98%), 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM·PF6, >98.5%), sodium azide (>99%), sodium hydroxide pellets (>97%) were purchased from Sigma-Aldrich (www.sigmaaldrich.com); chloroform (CHCl3) and methanol (MeOH) were purchased from PANREAC (www.albuslaboratorios.com). All reagents were used as received without further purification. Ultrapure water used throughout all experiments was purified through a Millipore system. Toothpaste, and coconut milk and syrup were purchased from a local supermarket.
Instrumentation
The AF4 analysis were performed with the commercially AF2000 (Postnova Analytics GmbH, Landsberg, Germany) model coupled online to a 21-angle MALS (PN3621, Postnova Analytics), and RI detectors (60% power). The average hydrodynamic diameter and size distribution of NC aqueous samples were calculated at room temperature, using a Malvern Zetasizer Nano ZSP ZEN5600 analyzer equipped with a 10 mW laser He-Ne (633 nm). A Vibracell™ 75,041 ultrasonic probe (750 W, 20 KHz, Bioblock Scientific, Illkirch, France) equipped with a 3 mm probe was employed for the dispersion of the suspensions. Infrared (IR) measurements were performed in a Shimadzu IRPRESTIGE-21 spectrometer equipped with an attenuated total reflectance (ATR) device. The pH of the media was measured with a Basic 20 pH-meter from Crison in all tests.
Preparation of nanocellulose
Synthesis of nanocellulose
The preparation of nanocellulose (NC) was described previously [27], but some modifications were included to perform an up-scale synthesis: Microcrystalline cellulose (MCC; 5 g) and NaBr (12.5 mg, 1.21 mmoL) were mixed with TEMPO radical (145 mg, 0.08 mmoL) in aqueous solution at room temperature. Then, NaClO (40 mL, 648.1 mmoL) were added dropwise onto the mixture while keeping constant the pH of the resulting solution at 10 using NaOH (1 M). After that, the mixture was stirred at room temperature until no changes in the pH were observed. Then, ethanol was added and the resulting suspension was then filtered and washed several times with water. Finally, the residue was precipitated using methanol, and dried under vacuum. A yield of 94% was obtained. Batch-to-batch reproducibility in terms of size characterization using DLS, resulted to be of 3.28% of relative standard deviation (n = 3).
Analytical method
Preparation of nanocellulose standards.
Samples for analysis were well-dispersed by an ultrasonic probe in order to assure the individualization of NC fibers in aqueous media. Amplitude of the ultrasonic vibrations was set at 30% of maximum nominal power for 30 min (cycles of 10 s on and 20 s off).
Extraction procedure
Extraction of NC from commercial products was carried out using the following protocol: 0.2 g of the samples (toothpaste, coconut milk or syrup) were accurately weighed into a clean, dry 5-mL Eppendorf tubes, mixed with CHCl3 (200 μL) and EDTA aqueous solution (3 mM, 5%v/v in MeOH, 400 μL) and, finally, centrifuged at 5000 rpm (3630 g) for 10 min. The aqueous extract containing NC was submitted to an ionic liquid assisted extraction procedure using BMIM·PF6 in combination with the cationic surfactant CTAC. After extraction, the supernatant was discarded, and the resulted solid was washed thrice with distilled water and finally precipitated using methanol. The resulting material was then dried at 80 °C in an oven and stored at room temperature until the subsequent AF4 analysis.
Fractionation and characterization
For AF4 measurements and DLS characterization, NC dispersions were prepared at concentrations of 5 and 2 g·L−1, respectively.
The membrane forming the accumulation wall was composed of regenerated cellulose with a 10 KDa cut-off.
All runs were performed using an aqueous mobile phase consisting of 0.2% of sodium azide, which has been demonstrated to be the most suitable for cellulose nanocrystals fractionation in preliminary assays. Along this work, various separation protocols were applied with different cross-flow sequences and gradients as well as detector flow rates. The applied conditions for a correct fractionation of NC are specified below.
During all settings, the cross-flow rate was kept constant at 1 mL·min−1 and detector flow rate was fixed at 0.5 mL·min−1 to reduced pressure fluctuations.
A focus flow of 0.9 mL·min−1 was first applied for 1.5 min prior the injection of the sample. All samples were injected at an injection flow of 0.6 mL·min−1 with a focus flow of 1 mL·min−1 for 5 min. During all the experiments 21 μL of the sample were injected. According to the NP diffusion coefficient, the analyte is aligned at a certain height over the accumulation wall.
For the separation process a four-step cross-flow sequence was used: a) 1 mL·min−1 flow constant for 7 min, b) power flow at 1 mL·min−1 for 40 min, c) ramped flow from 1 to 0.4 mL·min−1 for 40 min, d) ramped flow from 0.4 to 0.1 mL·min−1 for 10 min.
At the end of the fractionation process, a rinse step of 20 min with a cross flow of 1 mL·min−1 was used to minimize the possible contamination between samples.
Results and discusion
Sample preparation procedure
Selectivity arises from the separation of NC from other organic and inorganic compounds presented in complex samples is crucial. Figure 1 displays the extraction protocol of NC, which can be disclosed in two main steps.
Prior the extraction step, samples were treated with the chelating agent EDTA. Then, EDTA-treated samples were washed with CHCl3 in order to remove most of hydrophobic molecules and metal ions; this step is crucial to improve the efficiency of the detection method, avoiding problems on gelation of the fibers for the presence of salts.
Regarding the isolation of NC from the sample matrix, the extraction and preconcentration is based on the use of ionic liquids, especially those derived from the imidazolium group. The type of anions and the length of the alkyl group of the imidazolium ring were essential in the extraction step. Non-coordinating counterion such as hexafluorophosphate resulted to be critical for the extraction of NC from aqueous solutions. Regarding the cation effect, butyl pedant chain affixed to the quaternized nitrogen of the imidazolium provided the best separation as a result of the increase on the immiscibility to aqueous solutions. It is important to highlight the use of a cationic surfactant which displays two key roles in the extraction procedure; on the one hand, the surfactant has to avoid NC aggregation during the extraction, since it is of great important that no change in the size of the cellulosic fibers is produced during the sample preparation. On the other hand, the surfactant may assist in the NC transfer through the water/ionic liquid interface in the extraction. Thus, evaluation of different cationic surfactants (CTAB and CTAC) were studied in first place, observing that CTAC was able to extract the NC at higher extend than CTAB, possibly due to the sterically influence of the anion. The concentration of the CTAC (0.1–0.5 M) was also evaluated to allow the solubilisation of NC into the ionic liquid. NC were stabilized by a shell layer of the surfactant (at a concentration of 0.1 M) for the selective transfer to the ionic liquid phase, and then, precipitated for the subsequent analysis with AF4. The use of alcohol was essential for removing the ionic liquid to make more compatible the medium for the AF4 analysis. The extraction efficiency of the experimental procedure was quite acceptable, resulting in an average value of 80.9 ± 1.8% (n = 5).
Detection of nanocellulose with asymmetric flow field-flow fractionation
AF4 resulted to be suitable for the NC analysis, once extracted from the deodorant and coconut milk and syrup as indicated in the sample preparation. Importantly, preliminary experiments in AF4 were performed to find an effective AF4 method suitable for all types of NC. Thus, AF4 analysis of the native NC was subjected to different methods for optimizing the specific parameters and steps for removing the aggregation effect, loses of NPs and improving the fractionation.
An essential parameter to be specified for the correct analysis of the light scattering data is the differential index of refraction (dn/dc), which is proportional to the solute concentration but also depends on temperature and pressure. Since this parameter is not registered for NC in the literature, its determination is crucial, and thus, preliminary experiments were performed in first place. The dispersion of native NC was first thermostated prior the injection and then, flushed through the detector using a constant tip flow of 0.5 mL·min−1 The resulting differential refractive index value was derived to be 0.126 g·L−1.
AF4-MALS parameter optimization
The influence of the carrier liquid, sample load and the cross and detector flow rates were evaluated for the optimal fractionation and size analysis of NC.
For the mobile phase optimization, different carrier compositions were evaluated in order to choose the better fractionation for cellulose nanofibers. For that reason, native NC were well-dispersed in a variety of aqueous solutions with an ultrasonic tip and checked their stability by visualization and Zeta potential. On the one hand, the use of anionic surfactant, SDS, gave bad results in the fractionation process. On the other hand, better results were observed in ultrapure water and sodium chloride buffer although direct visualization of NC confirmed the partial aggregation of the fibers in the last medium, whereas in ultrapure water no aggregation was observed. DLS experiments corroborated the aggregation of NC, as depicted in Fig. S2; in fact, NC dispersions on 0.1 mol·L−1 aqueous solution containing NaCl partially aggregates after 20 min. Standing whilst NC in ultrapure water remains stable against aggregation for longer period of time, as confirmed by DLS.
Thus, all analyses were performed with NC dispersions in ultrapure water in presence of traces of azide to assure the absence of microorganisms in the equipment.
The sample load was varied from 1 to 10 g·L−1 (1, 2, 5 and 10 g·L−1). All experiments were performed in triplicate. Experimentally, 5 g·L−1 resulted to be the optimal value to get the best signal-to-noise ratio. That means that injection volume was of 21 μL. Higher sample concentration resulted in a distorted light scattering signal possibly due to the repulsion between particles situated in the same layer of the parabolic profile, and as a result, particles migrate to higher velocity streamlines of the longitudinal channel and elute earlier.
The pivotal step in the quality of the fractionation is the selection of the suitable cross flow rate, since it governs the equilibrium height of the NPs over the accumulation wall. Thus, the cross-flow rate was evaluated in a range from 3.5 to 0.5 ml·min−1, being 1 mL·min−1 the ideal chosen setup for establishing an equilibrium between the elution time and a reasonable resolution. With increasing cross flow rates the equilibration height over the accumulation wall decreases resulting in a longer retention time and a lower resolution.
The influence of the detector flow rate was also evaluated from 1 to 0.2 ml·min−1 with the purpose of obtaining a good signal and avoiding the dilution of the fractions. Thus, the optimal value was set up at 0.5 mL·min−1.
Characterization of native nanocellulose and nanocellulose from commercial products
After the extraction procedure of NC, we used AF4-MALS measurement and DLS for their size separation and characterization. However, it is of great importance the preservation of the functionalities and stability of NPs through the overall sample preparation procedure. Thus, the comparison of the structural characteristics of the fibers using IR spectroscopy was needed to assure the non-modification of their surface before and after the sample preparation.
As shown in Fig. S3, no significant structural changes in the cellulosic surface before and after the extraction of NC from commercial products were found, being their main characteristic peaks those at 3354 and 2905 cm−1 related to the OH stretching (hydrogen-bonded) and CH stretching modes, respectively, and the CH and OCH in-plane bending vibration modes at 1433 cm−1.
In addition, the Zeta potential of the native NC in ultrapure water was found to be −52.5 mV, as would expected for defibrillation and oxidation of MCC with TEMPO radial; the extracted NC displayed similar values (Fig. S4), indicating the preservation of the superficial charge and the well-dispersion of the nanofibers in the carrier liquid at the established concentration. Thus, it is expected lack of sample losses derived by the adsorption effect of the NPs onto the accumulation wall by electrostatic interactions because of the negatively charged the membrane chosen for the fractionation.
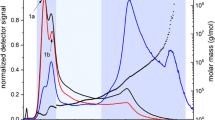
In general, during all measurements the retention ratio of the eluted NC gradually decreases as the particle size increases (normal mode) (Fig. 2). Interestingly, all the samples were adjusted to a Debye second order model, as expected for the type of NPs. In addition, all fractograms looked rather similar in terms of peak shape and elution time. Native NC was used as standard in the analysis of AF4-MALS, and the distribution of the major population was found to be in the range of 20–55 nm (Fig. 2a). Despite the fact that the AF4-MALS fractograms of the extracted NC from deodorant and coconut foodstuff (in Fig. 2b–c) displayed similar retention time, higher size distributions of the NPs (around 55–100 nm) compared to native NC were observed. In all cases, the majority of the sample is eluted at early elution times as a result of the faster diffusion time of small particles, which showed low light scattering intensities.
Figure 3 displays the size distribution derived from MALS of the native and extracted NC. It is clearly shown that native NC has different size distributions, being the major ones those around 37, 42, 52 and 62 nm (Fig. 3a). However, a trimodal population was found for NC extracted of deodorant, being around 52, 62 and 72 nm (Fig. 3b). The NPs from the coconut foodstuff are very different; a wide range distribution was observed from the milk (Fig. 3c) whilst the syrup exhibited just two main populations around less than 40 nm and above 100 nm (Fig. 3d).
Therefore, it is possible to know the hydrodynamic diameter of the extracted NC by the use of DLS, as depicted in Fig. 4. The samples showed polydispersity and similar trends in particle size distribution, with higher hydrodynamic ratio measured by DLS. In fact, it can be stated that smaller hydrodynamic diameters were observed for the NC extracted from coconut syrup, which is in accordance with the AF4 results (Fig. 3d).
The extraction procedure and the appropriate method of AF4-MALS for NC detection and characterization enable real-sample monitoring of consumer products.
Although the importance of detecting and size-characterizing NPs in complex matrices, this method lacks of quantitative information; this limitation could be implemented by the use of a fraction collector in the AF4 equipment coupled to an additional detector like DLS.
Conclusions
An experimental setup for detecting NC in cosmetics and foodstuff has been established based on a sample preparation based on ionic liquid-assisted liquid-liquid extraction and a detection with an AF4 method. On the one hand, the extraction of NC from commercial products into the ionic liquid BMIM·PF6 was successfully achieved in the presence of CTAC. The integrity of the nanomaterial is corroborated with the surface, size and charge characterization using FT-IR spectroscopy, DLS and Zeta potential measurements. AF4 coupled with MALS and RI detectors was demonstrated to be a powerful analytical tool for the size characterization of NC with high polydispersity. A general protocol for the analysis and characterization of NC by a unique AF4 method appeared appropriate to gain knowledge over particle size in the range of 30–100 nm; the method is applied to all extracted NC from toothpaste and coconut foodstuff. The rod length distribution approximately spanned from 40 to 70 nm in the case of toothpaste, from 70 to 94 nm in coconut milk and about 40 and 100 nm in coconut syrup.
Although AF4 can be combined with multiple detectors for subtracting multidimensional information about NPs in one single analysis, this paper only explores MALS and RI detectors for qualitative analysis. The possibility to connect AF4 with other sensitive detectors might provide a more complete inside in the NP determination in terms of limit of detection/quantification, composition and size. A combination of DLS and MALS in the AF4 instrumentation should be of great importance for the online downstream analysis of the fractions suitable for obtaining important morphological information about NC.
The easy sample preparation for extracting the NC from a variety of consumer products gave expectations about their usefulness for future applications in nanoparticle detection.
References
Hannon JC, Kerry J, Cruz-Romero M, Morris M, Cummins E (2015) Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends Food Sci Technol 43:43–62
Fortunati E, Peltzer M, Armentano I, Jimenez A, Kenny JM (2013) Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J Food Eng 118(1):117–124
Gama M, Gatenholm P, Klemm D (2012) Bacterial nanocellulose: a sophisticated multifunctional material; Ed.; CRC Press
Dhar P, Bhardwaj U, Kumar A, Katiyar V (2014) In food additives and packaging; Komolprasert, V.; Turowski, P., Ed.; Am Chem Soc. 17;197–239.
Morais JP, Rosa MF, de Souza F, Nascimento LD, do Nascimento DM, Cassales AR (2013) Extraction and characterization of nanocellulose structures from raw cotton isolation and characterization of cellulose nanocrystals from Agave angustifolia fibre. Carbohydr Polym 91(1):229–235.
Lani NS, Ngadi N, Johari A, Jusoh M (2014) Isolation, characterization and application of nanocellulose from oil palm empty fruit bunch fiber as nanocomposites. J Nanomater 3:1–9
Kargarzadeh H, Ahmad I, Abdullah I, Dufresne A, Zainudin SY, Sheltami RM (2012) Effects of hydrolysis conditions on the morphology, crystallinity and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 19(3):855–866
Yongvanich NJ (2015) Isolation of nanocellulose from Pomelo fruit fibers by chemical treatments, journal of natural fibers. Nat Fibers 12(4):323–331
Johar N, Ahmad I, Dufresne A (2012) Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crop Prod 37(1):93–99
Abd Hamid SB, Karim Z, AliGreen E (2014) Catalytic approach for the synthesis of functionalized nanocellulose from palm tree biomass. Adv Mater Res 925:57–61
Andrade DRM, Mendonça MH, Helm CV, Magalhães WLE, de Muniz GIB, Kestur SG (2015) Assessment of nano cellulose from peach palm residue as potential food additive: part ii: preliminary studies. J Food Sci Technol 52(9):5641–5650
Salas C, Nypelö T, Rodriguez-Abreu C, Carrillo C, Rojas OJ (2014) Nanocellulose properties and applications in colloids and interfaces. J Colloid Interface Sci 19:383–396
Andersson M, Wittgren B, Wahlund K (2001) Ultrahigh molar mass component detected in ethylhydroxyethyl cellulose by asymmetrical flow field-flow fractionation coupled to multiangle light scattering. Anal Chem 73:4852–4861
Sánchez-García L, Bolea E, Laborda F, Cubel C, Ferrer P, Gianolio D, da Silva I, Castillo JR (2016) Size determination and quantification of engineered cerium oxide nanoparticles by flow field-flow fractionation coupled to inductively coupled plasma mass spectrometry. J Chromatogr A 1438:205–215
Guan X, Cueto R, Russo P, Qi Y, Wu Q (2012) Asymmetric flow field-flow fractionation with multiangle light scattering detection for characterization of cellulose nanocrystals. Biomacromolecules 13(9):2671–2679
Nguyen TM, Pettibone JM, Gigault J, Hackley VA (2016) In situ monitoring, separation, and characterization of gold nanorod transformation during seed-mediated synthesis. Anal Bioanal Chem 408(9):2195–2201
Astefanei A, Núñez O, Galceran MT, Kok WT, Schoenmakers PJ (2015) Aggregation behavior of fullerenes in aqueous solutions: a capillary electrophoresis and asymmetric flow field-flow fractionation study. Anal Bioanal Chem 407:8035–8045
Müller D, Cattaneo S, Meier F, Welz R, de Vries T, Portugal-Cohen M, Antonio DC, Cascio C, Calzolai L, Gilliland D, de Mello A (2016) Inverse supercritical fluid extraction as a sample preparation method for the analysis of the nanoparticle content in sunscreen agents. J Chromatogr A 1440:31–36
Lee WC, Lee BT, Lee S, Hwang YS, Jo E, Eom IC, Sang-Woo L, Soon-Oh K (2016) Optimisation, evaluation and application of asymmetrical flow field-flow fractionation with single particle inductively coupled plasma mass spectrometry (SP-ICP-MS) to characterise silver nanoparticles in environmental media. Microchem J 129:219–230
Pettibone JM, Liu J (2016) In situ methods for monitoring silver nanoparticle sulfidation in simulated waters. Environ Sci Technol 50(20):11145–11153
Meisterjahn B, Wagner S, von der Kammer F, Hennecke D, Hofmann T (2016) Silver and gold nanoparticle separation using asymmetrical flow-field flow fractionation: influence of run conditions and of particle and membrane charges. J Chromatogr A 1440:150–159
Barahona F, Ojea-Jimenez I, Geiss O, Gilliland D, Barrero-Moreno J (2016) Multimethod approach for the detection and characterisation of food-grade synthetic amorphous silica nanoparticles. J Chromatogr A 1432:92–100
Schimpf ME, Caldwell K, Giddings JC (2000) Field-flow fractionation handbook. Wiley, New York
Gimbert LJ, Andrew KN, Haygarth PM, Worsfold PJ (2003) Environmental applications of flow field-flow fractionation (FIFFF). Trends Anal Chem 22(10):615–633
Messaud FA, Sanderson RD, Runyon JR, Otte T, Pasch H, Williams SKR (2009) An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog Polym Sci 34(4):351–368
Grumezescu AM (2016) In self-assembly of nanobiomaterials. Aplications of nanobiomaterials; Ioelovich, M., Ed.; Elsevier, volume 1, pp 243–281.
Saito T, Nishiyama Y, Putaux JL, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Acknowledgements
The authors would like to express their gratitude to the Spanish Ministry of Innovation and Science for funding Project CTQ2014-52939R. We also want to thank the Fine Chemistry Institute of the University of Córdoba for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 276 kb)
Rights and permissions
About this article
Cite this article
Ruiz-Palomero, C., Laura Soriano, M. & Valcárcel, M. Detection of nanocellulose in commercial products and its size characterization using asymmetric flow field-flow fractionation. Microchim Acta 184, 1069–1076 (2017). https://doi.org/10.1007/s00604-017-2106-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2106-6