Abstract
We describe the use of a nanocomposite consisting of graphene and β-cyclodextrin (β-CD) which was used to modify a glassy carbon electrode (GCE) to serve as a matrix for immobilization of hemoglobin (Hb). The composite was characterized by scanning electron microscopy, UV-vis and FTIR spectroscopy. The modified electrode displays an enhanced and well-defined quasi reversible peaks for the heme protein at a formal potential of −0.284 V (vs. Ag/AgCl). The direct electrochemistry of Hb is strongly enhanced at this modified electrode compared to electrodes not modified with graphene or β-CD. The heterogeneous electron transfer rate constant (Ks) is 3.18 ± 0.7 s−1 which indicates fast electron transfer. The biosensor exhibits excellent electrocatalytic activity towards the reduction of bromate, with a linear amperometric response in the 0.1 to 176.6 μM concentration range at a working voltage of −0.33 V. The sensitivity is 3.39 μA μM−1 cm−2, and the detection limit is 33 nM. The biosensor is fast, selective, well repeatable and reproducible, and therefore represents a viable platform for sensing bromate in aqueous samples.

Electrochemical sensing of bromate at hemoglobin immobilized on graphene and β-cyclodextrin composite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of chemical contaminants is known to have a serious impact on human health even if present in very low concentrations only in drinking water [1]. In particular, bromate is well-known oxyhalide obtained as byproduct of disinfection of drinking water via ozonation. It can easily contaminate drinking water [2, 3]. Bromate is also classified as one of a carcinogenic and genotoxic compound by the International Agency of Research on Cancer [4]. In addition, the US Environmental Protection Agency (EPA), the European Council (EC) and the World Health Organization (WHO) are stated that a maximum permissible level of bromate in drinking water is 10 μg L−1 [4]. The excess level of bromate in drinking water leads to the damage of kidneys, nervous system and renal cell tumors [5]. Hence, the accurate and low level detection of bromate is of great importance in real samples for human health. So far, the different analytical methods have been developed for sensitive detection of bromate, including spectrophotometry, chromatographic methods, florescent and electrochemical methods [6–9]. However, the electrochemical methods are widely used for the detection of bromate owing to its simplicity along with high sensitivity and selectivity than that of traditional spectrophotometry and chromatographic methods [10].
Hemoglobin (Hb) is a redox metalloprotein, plays an important role in oxygen transport and storage in mammalian blood [11]. Hb is an ideal redox metalloprotein for the construction of biosensors due to its low cast and availability [12]. In addition, Hb is widely used for the detection of hydrogen peroxide, nitrite, trichloroacetic acid and bromate, due to its high specificity [13]. However, the direct immobilization and direct electron transfer of Hb on the conventional electrodes are still challenging, thus the modified electrodes have been used for the immobilization of Hb [14]. Recent years, different nano and micro materials have been widely used for immobilization of Hb on the electrode surface [15]. Graphene or reduced graphene oxide is a two-dimensional sp2 hybridized carbon nanomaterials, displays high mechanical, electrical and thermal properties than that of other carbon nanomaterials [16]. Furthermore, graphene and reduced graphene oxide (RGO) based composites are continuously used for the construction of electrochemical biosensors [17–19]. Though, the special properties of graphene highly abridged in RGO when compared to the properties of pristine graphene [9]. Hence, the pristine graphene is an alternative choice to prolong its special properties to the biosensors fabrication. However, pristine graphene can be easily agglomerates to graphite in aqueous solutions due to the π stacking of individual graphene sheets, hence different materials have been used to prevent the agglomeration of graphene sheets and enrich the biocompatibility [20]. For instance, the supramolecular adducts, surfactants and polymers are predominantly used to prevent the restacking of graphene sheets and increase the dispersing ability of graphene in aqueous solutions [21]. In particular, β-cyclodextrin (β-CD) is an oligosaccharide, composed with 6–8 glucopyranoside units and form host-guest complexes with hydrophobic molecules owing to its unique properties [22]. Thus, we have used β-CD as a dispersing agent and stabilizer for graphene. The resulting graphene/β-CD composite showed an enhanced biocompatibility than pristine graphene. In addition, graphene/β-CD composite provides a suitable micro environment for immobilization of Hb. However, for the first time we have used β-CD as a dispersing agent for pristine graphene and used as an immobilization matrix for Hb, which greatly enhanced the direct electron transfer of Hb towards the electrode surface.
We report an amperometric bromate biosensor based on the direct electrochemistry of immobilized Hb at graphene/β-CD composite for the first time. The graphene/β-CD/Hb modified electrode shows an enhanced and well-defined quasi reversible peaks for Hb compare to other modified electrodes. Subsequently, the resulting biosensor was used to determine the bromate by amperometric method. The selectivity and practicability of the biosensor was studied and discussed in detail. The present biosensor is simple and can detect the bromate in sub-millimolar levels compared with previously reported bromate sensors.
Experimental
Chemicals
Graphene nanopowder (8 nm flakes, product number UR-GNAPHENE) was purchased from UniRegion Bio-Tech, Taiwan. β-cyclodextrin, hemoglobin from bovine blood, potassium bromate, dopamine, ascorbic acid, glucose and all other chemicals were purchased from Sigma Aldrich (http://www.sigmaaldrich.com/taiwan.html) and used as received. The supporting electrolyte 0.05 M phosphate buffer pH 7 was prepared by using 0.05 M Na2HPO4 and NaH2PO4 in doubly distilled water. All the other chemicals were of analytical grade and the solutions were prepared using doubly distilled water without any further purification.
Apparatus
Cyclic voltammetry (CV) and amperometric i-t experiments were performed by CHI 1205b and CHI 750a electrochemical work stations from CH instruments. Scanning electron microscopy (SEM) was performed using Hitachi S-3000 H electron microscope. Fourier transform infrared spectroscopy (FTIR) was carried out using the Thermo Scientific Nicolet iS10 instrument. Raman spectra were recorded using a Raman spectrometer (Dong Woo 500i, Korea) equipped with a charge-coupled detector. UV-Vis spectroscopy was performed by a Hitachi U-3300 UV spectrophotometer. A typical three-electrode system was used for the electrochemical experiments, where glassy carbon electrode (GCE) as a working electrode, saturated Ag/AgCl as a reference electrode and platinum electrode as the auxiliary electrode. Amperometric i–t measurements were performed using a PRDE-3 A (AS distributed by BAS Inc. Japan) rotating ring disc electrode (RDE) and the electrochemically active surface area (EASA) was calculated as 0.082 cm2 by CV, as we reported early [23].
Preparation of graphene/β-CD composite and immobilization of Hb
The graphene/β-CD composite was prepared by the addition of 10 mg graphene into the 10 mL of β-CD aqueous solution and the mixture was ultra-sonicated for 30 min at room temperate. The β-CD aqueous solution was prepared by dispersing of 10 mg of β-CD into the 10 mL of distilled water with the aid of ultra-sonication about 10 min. The obtained graphene/β-CD composite was centrifuged to remove the excess amount of graphene from the graphene/β-CD composite. The obtained graphene/β-CD composite was re-dispersed into the water and used for further characterization and electrochemical studies. The graphene/β-CD composite modified electrode was prepared by drop coating of 8 μL of graphene/β-CD dispersion on pre-cleaned GCE and dried in a room temperature. The graphene and β-CD modified electrodes were also independently prepared by similar method without β-CD and graphene. The graphene and β-CD dispersions were prepared by dispersing of graphene (1 mg mL−1) in dimethylformamide and β-CD (1 mg mL−1) in water with the aid of ultra-sonication about 15 min. The RGO/β-CD composite modified electrode was prepared by electrochemical reduction of graphene oxide/β-CD composite in pH 5 solution at applied potential of −1.4 V for 300 s. The biosensor was prepared by drop coating of 8 μL of Hb solution (5 mg mL−1) on the graphene/β-CD composite modified GCE and dried at room temperature. The graphene/β-CD/Hb modified GCE was gently rinsed a few times with doubly distilled water to remove the loosely bounded Hb. The Hb stock solutions were prepared using 0.05 M phosphate buffer pH 7 and the electrochemical experiments were performed in N2 atmosphere at room temperature. The biosensor was stored in 0.05 M phosphate buffer pH 7 at 4 °C when not in use.
Results and discussion
Choice of materials
We have chosen Hb as a model redox protein for the construction of biosensor, due to its known structure, commercial availability, and relatively higher stability than that of other iron redox proteins, such as myoglobin and cytochrome C. In addition, Hb has high specificity towards the detection of bromate compared to commonly available well-known cobalt complex modified electrodes. We have used graphene and β-CD for the composite preparation and it has two main advantages. First, pristine graphene is the best choice to prolong all the special properties of graphene, while, the special properties of graphene are much deduced in the RGO and graphite based composites [9]. Second, β-CD prevents the agglomeration of graphene sheets and improves the biocompatibility of graphene in graphene/β-CD composite, which provides a suitable micro environment for immobilization of Hb. The strong interaction between the hydroxy groups of β-CD and edge planes of graphene nanosheets is resulting to the formation of stable graphene/β-CD composite [9]. In addition, β-CD component in graphene/β-CD composite greatly enhanced the electrochemical behavior of Hb via strong host–guest interaction between the β-CD and graphene that efficiently translates into an electrochemical signal to the electrode [9].
Characterizations
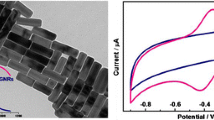
Figure 1 displays the SEM images of un-modified (a), graphene (b), β-CD (c) and graphene/β-CD composite (d) modified electrodes. The unmodified electrode shows typical clean morphology for GCE. While, the graphene modified electrode reveals its typical flake morphology with association of bundle of nano graphene sheets. On the other hand, β-CD modified electrode shows the typical sheet morphology, which is more consistent with our previously reported method. The SEM image of graphene/β-CD composite modified electrode shows the crumbled morphology, where graphene nano sheets were entrapped by β-CD sheets (Fig. 1d). The polar hydroxyl groups on β-CD can easily interact with the edge planes of the graphene sheets, which are resulting to the formation of graphene/β-CD composite.
The formation of graphene/β-CD composite was further confirmed by FTIR spectroscopy. It is well-known that FTIR spectroscopy is widely used to confirm the functional groups and study the interaction between the compounds.
Figure 2a shows the FTIR spectrum of graphene (a), β-CD (b) and graphene/β-CD composite (c). The FTIR spectrum of graphene did not show obvious peaks in the fingerprint region, while graphene/β-CD composite shows three distinct peaks around 3382, 2965 and 1015 cm−1, which corresponds to the O–H (from water), C–H stretching vibration and C–O–C groups from the β-CD, which is similar to the FTIR spectrum of β-CD, as reported early [24]. The result confirms that the polar hydroxyl groups of β-CD strongly interact with edge of the graphene sheets and resulting to the formation of graphene/β-CD composite.
UV-Vis spectroscopy is commonly used to confirm the Soret absorption band of proteins. The presence of Hb on graphene/β-CD/Hb modified electrode was confirmed by UV-Vis spectroscopy. We have used Indium tin oxide (ITO) electrode for UV-Vis spectroscopy studies, in which graphene/β-CD, Hb and graphene/β-CD/Hb composite was directly coated on the ITO electrode surface. Figure 2b shows UV-Vis spectrum of graphene/β-CD composite (a), native Hb (b) and graphene/β-CD/Hb composite (c). The UV-Vis spectra of graphene/β-CD composite did not show obvious Soret band, while pure Hb shows the Soret band at 409 nm, which is very similar to the previously reported Soret absorption band of native Hb [25]. The graphene/β-CD/Hb composite shows the Soret band at 410.0 nm and the observed value is very close to Soret absorption band for native Hb [25]. These results indicate that the native morphology of Hb is retained in the graphene/β-CD composite modified electrode.
Raman spectroscopy was used for further characterization of graphene and graphene/β-CD composite, since Raman spectroscopy is widely used to confirm the transformation of carbon nanomaterials. Figure 3 shows the Raman spectra of graphene (red color) and graphene/β-CD composite (black color). The graphene and graphene/β-CD composite exhibit a weak D and strong G bands at 1389 and 1589 cm−1 and 1402 and 1590 cm−1, respectively. While, the enhanced 2D band of graphene and graphene/β-CD composite was observed at 2723 and 2726 cm−1. The graphene/β-CD composite has showed an enhanced D, G and 2D bands compared to the D, G and 2D bands observed for graphene, which is due to the strong interaction between the β-CD rims with edge planes of graphene.
The 2D/G intensity ratio of graphene and graphene/β-CD composite was calculated as 1.11 and 1.14, which indicates that few layer structure of graphene in graphene/β-CD composite. Usually the monolayer graphene will have the ratio of 2D/G intensity about 2.5 and few layer graphene have a 2D/G intensity ratio about 1.0.
Direct electrochemistry of Hb
Figure 4 shows the CV response obtained at Hb immobilized on bare (a), graphene (b), β-CD (c) and graphene/β-CD composite (d) modified GCEs in N2 saturated 0.05 M phosphate buffer pH 7 at a scan rate of 100 mV s−1. The CV experiments were performed in the potential range between 0.1 to −0.8 V. The bare and β-CD modified electrodes does not show any apparent direct electrochemical behavior to Hb, which indicates that these kind of electrodes are not suitable for immobilization of Hb. The graphene modified electrode showed a weak redox couple at the formal potential (E0) of −0.169 V with a peak to peak separation (∆Ep) of 0.102 V. On the other hand, Hb immobilized graphene/β-CD modified electrode shows a pair of well-defined quasi redox couple at the E0 of −0.284 V, which is due to the direct electrochemical behavior of heme redox couple (FeII/FeIII) in Hb [26–28].
The ∆Ep of graphene/β-CD/Hb modified electrode was calculated as 54 mV, which is quite lower than graphene/Hb modified electrode. The result indicates the enhanced direct electron transfer of Hb on graphene/β-CD composite modified electrode than that of other modified electrodes. The graphene/β-CD composite is providing a suitable micro environment for immobilization of Hb and resulting to the enhanced direct electron transfer towards the electrode surface. We have also investigated the direct electron transfer of graphene/β-CD/Hb modified electrode in N2 saturated 0.05 M phosphate buffer pH 7 at a scan rate of 100 mV s−1 and the results are shown in Fig. S1. A pair of well-defined quasi redox couple for Hb was observed with E0 of −0.284 V (Fig. S1a). However, the observed redox peak currents are lower than those observed at graphene/β-CD/Hb modified electrode (Fig. S1b). Hence, graphene/β-CD composite modified electrode is more suitable for Hb immobilization than that of RGO/β-CD composite modified electrode.
The effect of scan rate on the electrochemical behavior of graphene/β-CD/Hb electrode was studied by CV. Figure 5a shows CV response obtained at graphene/β-CD/Hb electrode in N2 saturated 0.05 M phosphate buffer pH 7 at different scan rates from 20 to 200 mV s−1. It can be seen that the anodic and cathodic peak current of Hb increases with increasing the scan rates, while the anodic and cathodic peak potentials were slightly shifted towards the positive and negative potentials upon increasing the scan rates from 20 to 200 mV s−1. The anodic and cathodic current response of Hb was linear over the scan rates from 20 to 200 mV s−1 with the correlation coefficient (R2) of 0.9968 and 0.9946, respectively (Fig. 5b). The result indicates that the redox behavior of Hb is controlled by surface confined electrochemical process [27]. The apparent heterogeneous electron transfer rate constant (Ks) of graphene/β-CD/Hb modified electrode was estimated as 3.18 ± 0.7 s−1 using Laviron Eq. (1) for surface-controlled electrochemical system [29].
Where ΔEp = peak-to-peak separation of the redox couple (the scan rate was chosen when the ΔEp is equal or more than 200 mV), n = 1, α is assumed to be 0.5 and all other symbols are usual meanings. The calculated Ks value is much higher than the previously reported Hb immobilized modified electrodes [28–31]. The result validates that graphene/β-CD composite modified electrode greatly facilitate the direct electron transfer of Hb towards the electrode surface.
The effect of pH on the redox electrochemical behavior of graphene/β-CD/Hb modified electrode was studied in N2 saturated different pH using CV and the results are shown in Fig. S2A. It can be seen that a well-defined redox couple of Hb was observed in pH from 3 to 9. The anodic and cathodic peak potential of Hb shift towards positive and negative direction upon decreasing and increasing the pH. As shown in Fig. S2B, the E0’ of Hb had a linear dependence over the pH range from 3 to 9 with a slope of −51.6 mV pH−1 and a correlation coefficient of 0.9979. The observed slope value (−51.6 mV pH−1) is close to the theoretical value of the Nernst equation for an equal number of proton and electron transfer reversible electrochemical process [26]. Hence, the direct electrochemistry of graphene/β-CD/Hb modified electrode is involving of an equal number of protons (H+) and electrons (e−) transferred electrochemical reaction.
Amperometric determination of bromate
The electrocatalytic activity of graphene/β-CD/Hb modified electrode towards bromate was studied using CV. Fig. S3A shows CV response of graphene (a), β-CD (b), graphene/β-CD (c) and graphene/β-CD/Hb (d) modified electrodes in 100 μM bromate containing N2 saturated 0.05 M phosphate buffer pH 7 at a scan rate of 100 mV s−1. It can be seen that graphene and β-CD modified electrodes show less electrocatalytic activity towards the reduction of bromate, while graphene/β-CD composite modified electrode shows an enhanced reduction peak current for bromate at the potential of −0.427 V. In addition, the observed reduction peak current was 2 folds higher than that observed in graphene and β-CD modified electrodes. On the other hand, graphene/β-CD/Hb modified electrode shows a sharp reduction peak at −0.367 V in the presence of bromate. Furthermore, Hb immobilized graphene/β-CD composite modified electrode shows 2 folds enhanced reduction peak current with a positive potential shift towards the detection of bromate than that of graphene/β-CD composite modified electrode. The result indicates that graphene/β-CD/Hb modified electrode is more suitable for the determination of bromate than that of other modified electrodes. The electrocatalytic reduction of bromate with different concentrations (50–250 μM) was studied using graphene/β-CD/Hb modified electrode by CV and the results are shown in Fig. S3B. The reduction peak current of Hb was increased with the addition of different concentrations of bromate from 50 to 250 μM. The increase in the reduction peak current and decrease in the peak potential is considered as electrocatalysis. The result indicates that Hb immobilized graphene/β-CD electrode has good electrocatalytic activity towards the reduction of bromate. The effect of Hb amount on graphene/β-CD composite towards the detection of bromate was optimized and the 8 μL of Hb drop coated composite was found to give better results (Fig. S3B inset). The electrochemical reduction of bromate by graphene/β-CD/Hb modified electrode can be written by Eqs. 2 and 3, as reported early [13].
Figure 6a shows the typical amperometric i-t response obtained at graphene/β-CD/Hb modified RDE upon addition of different concentrations of bromate (0.1–186.6 μM) into the N2 saturated constantly stirred 0.05 M phosphate buffer pH 7. The electrode working potential held at −0.33 V. It can be seen that a well-defined and stable amperometric response was observed for addition of 0.1 and 1 μM of bromate (Fig. 6a inset), which indicates the high electro-reduction ability of the graphene/β-CD/Hb modified electrode. The biosensor reaches its steady state current within 3 s, indicating the fast electrocatalytic reduction of bromate by immobilized Hb on graphene/β-CD.
a Amperometric i-t response of graphene/β-CD/Hb modified RDE for the addition of different concentration of bromate from 0.1 to 186.6 μM into the constantly stirred N2 saturated 0.05 M phosphate buffer pH 7 at a working potential of −0.33 V. Inset shows the enlarged amperometric response up to 2.2 μM addition of bromate. b The calibration plot for amperometric current response vs. [bromate]. Error bar is related to the standard deviation for 3 measurements
As shown in Fig. 6b, the reduction peak currents of bromate was linear over the concentration ranging from 0.1 to 176.6 μM. The linear regression equation can be written from the fitted values from current vs. [bromate] as I (μA) = 0.2777 (μM) + 0.7197 with a correlation coefficient of 0.9947. The sensitivity was calculated from the fitted linear regression equation as 3.39 μA μM−1 cm−2. The limit of detection (LOD) was found to be 0.033 μM based on S/N = 3. In order to demonstrate the advantage of the biosensor, the analytical performance of the graphene/β-CD composite modified electrode was compared with previously reported modified electrodes for detection of bromate and comparison results are shown in Table 1. Table 1 clearly reveals that the graphene/β-CD/Hb modified electrode showed high sensitivity and low LOD than that of previously reported bromate sensors [10, 13, 31–35]. In addition, the linear response range of the graphene/β-CD composite modified electrode is comparable with the previously reported modified electrodes, as shown in Table 1. The results confirmed that the graphene/β-CD/Hb modified electrode can be used for sensitive determination of bromate.
Selectivity and operational stability of the graphene/β-CD/Hb modified electrode
The amperometric i-t method was used to evaluate the selectivity of the modified electrode under working conditions similar to those in Fig. 6a. Fig. S4A shows the amperometric i-t response of the modified RDE for the addition of 2 μM bromate (a) and 500 μM of Fe2+ (b), Fe3+ (c), Mg2+ (d), Ni2+ (e) Ca2+ (f), Cl− (g), Br− (h) and I− (i) into the N2 saturated constantly stirred 0.05 M phosphate buffer pH 7 at working potential of −0.33 V. It can be seen that 500 μM additions of metal ions did not cause an amperometric response of the modified electrode, which indicates the high selectivity of the biosensor. We have also evaluated the selectivity of the biosensor in the presence of biologically active compounds. Fig. S4B shows the amperometric i-t response on addition of 2 μM bromate (a) and 200 μM of dopamine (b), uric acid (c), nitrite (d), nitrate (e) and iodate (f) into the N2 saturated constantly stirred 0.05 M phosphate buffer pH 7 at working potential of −0.33 V. A stable and sharp amperometric response observed for the addition of 2 μM bromate, while 100 fold additions of biologically active compounds and nitrite, nitrate and iodate did not show any apparent response. The above results confirmed that the modified electrode can be used for selective detection of bromate in the presence of common metal ions and biologically active compounds. The high selectivity of the bromate is due to the strong interaction between the bromate ions and reduced for redox active center of Hb, as described in Eq. 3. The operational stability of the electrode for the response to 25 μM bromate was investigated by amperometry and the results are shown in Fig. S4C. The experimental conditions are similar as of in Fig. 6a. The electrode retains 98.1 % of its initial amperometric current response after continues run up to 1800 s in 25 μM bromate containing the N2 saturated constantly stirred 0.05 M phosphate buffer pH 7. The result indicates that the high operational stability of the fabricated biosensor. Its stability was investigated for up to 10 h by CV, which was stored in water prior to analysis. Fig. S5 shows the CV response for initial (a) and every 1 h up to 10 h (b–j) in N2 saturated 0.05 M phosphate buffer pH 7 at a scan rate of 100 mV s−1. It can be seen that the redox peak current response of Hb was almost unchanged when stored in water up to 10 h, which indicates that Hb was firmly attached on graphene/β-CD composite and resulting to the high storage stability.
Practicality, repeatability and reproducibility of the graphene/β-CD/Hb modified electrode
The practical applicability of the electrode was evaluated for the detection of bromate in different water samples by amperometric method. The experimental conditions are similar as of in Fig. 6a. The standard addition method was used to calculate the recovery of bromate in different water samples and the recovery results are shown in Table 2. The electrode indicated the absence of bromate in plain tap and drinking water samples, while the average recoveries of bromate in spiked water samples was about 96.0 %. The repeatability and reproducibility of the biosensor were evaluated by CV and the experimental conditions are similar to those in Fig. S3A. A single electrode for 6 successive measurements was found with the RSD of 4.6 % for the detection of 100 μM bromate. Six independently prepared electrodes were applied to the detection of 100 μM bromate and showed an RSD of 3.9 %. The result indicates that the composite electrode has good repeatability and reproducibility for the detection of bromate.
Conclusions
The direct electrochemistry of Hb was successfully realized on graphene/β-CD composite modified electrode. The graphene/β-CD/Hb modified electrode showed an enhanced direct electrochemistry when compared to the response observed at RGO/β-CD/Hb modified electrode. The strong host–guest interaction ability between the β-CD and graphene is resulting into the enhanced electrochemical signal of Hb towards the electrode surface. Compared to other available modified electrodes, the new electrode showed wide linear response, high sensitivity and low LOD for the determination of bromate. The good recovery and selectivity of the Hb immobilized composite authenticates that it can be used for selective detection of bromate in water samples. However, the biosensor has some limitations, such as continues sensing of bromate in waste water samples and working at high temperatures (above 50 °C). The biosensor sill needed to address these problems in the future and further works are in under way in our lab.
References
Marák J, Staňová A, Vaváková V, Hrenáková M, Kaniansky D (2012) On-line capillary isotachophoresis–capillary zone electrophoresis analysis of bromate in drinking waters in an automated analyzer with coupled columns and photometric detection. J Chromatogr A 1267:252–258
Saraji M, Khaje N, Ghani M (2014) Cetyltrimethylammonium-coated magnetic nanoparticles for the extraction of bromate, followed by its spectrophotometric determination. Microchim Acta 181:925–933
Oliveira SM, Oliveira HM, Segundo MA, Rangel AOSS, Lima JLFC, Cerdà V (2012) Automated solid-phase spectrophotometric system for optosensing of bromate in drinking waters. Anal Methods 4:1229–1236
Mao R, Zhao X, Lan H, Liu H, Qu J (2015) Graphene-modified Pd/C cathode and Pd/GAC particles for enhanced electrocatalytic removal of bromate in a continuous three-dimensional electrochemical reactor. Water Res 77:1–12
Zhang J, Yang X (2013) A simple yet effective chromogenic reagent for the rapid estimation of bromate and hypochlorite in drinking water. Analyst 138:434–437
Romele L (1998) Spectrophotometric determination of low levels of bromate in drinking water after reaction with fuchsin. Analyst 123:291–294
Cavalli S, Polesello S, Valsecchi S (2005) Chloride interference in the determination of bromate in drinking water by reagent free ion chromatography with mass spectrometry detection. J Chromatogr A 1085:42–46
Snyder SA, Vanderford BJ, Rexing DJ (2005) Trace analysis of bromate, chlorate, iodate, and perchlorate in natural and bottled waters. Environ Sci Technol 39:4586–4593
Guo Y, Guo S, Ren J, Zhai Y, Dong S, Wang E (2010) Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: synthesis and host − guest inclusion for enhanced electrochemical performance. ACS Nano 4:4001–4010
Papagiannia GG, Stergioua DV, Armatasb GS, Kanatzidisc MG, Prodromidis MI (2012) Synthesis, characterization and performance of polyaniline–polyoxometalates (XM12, X = P, Si and M = Mo, W) composites as electrocatalysts of bromates. Sensors Actuators B Chem 173:346–353
Qi H, Zhang C, Li X (2006) Amperometric third-generation hydrogen peroxide biosensor incorporating multiwall carbon nanotubes and hemoglobin. Sensors Actuators B Chem 114:364–370
Li J, Mei H, Zheng W, Pan P, Sun XJ, Li F, Guo F, Zhou HM, Ma JY, Xu XX, Zheng YF (2014) A novel hydrogen peroxide biosensor based on hemoglobin-collagen-CNTs composite nanofibers. Colloids Surf B 118:77–82
Vilian ATE, Chen SM, Kwak CH, Hwang SK, Huh YS, Han YK (2016) Immobilization of hemoglobin on functionalized multiwalled carbon nanotubes-Poly-L-Histidine-zinc oxide nanocomposites toward the detection of bromate and H2O2. Sensors Actuators B 224:607–617
Xu J, Liu C, Wu Z (2011) Direct electrochemistry and enhanced electrocatalytic activity of hemoglobin entrapped in graphene and ZnO nanosphere composite film. Microchim Acta 172:425–430
Zhao H, Ji X, Wang B, Wang N, Li X, Ni R, Ren J (2015) An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-β-cyclodextrin/Prussian bluechitosan nanocomposites for organophosphorus pesticides detection. Biosens Bioelectron 65:23–30
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183
Khan M, Tahir MN, Adil SF, Khan HU, Siddiqui MRH, Al-warthan AA, Treme W (2015) Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A 3:18753–18808
Pumera M, Ambrosi A, Bonanni A, Chng ELK, Poh HL (2010) Graphene for electrochemical sensing and biosensing. TrAC Trends Anal Chem 29:954–965
Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanalysis 22:1027–1036
Wu S, He Q, Tan C, Wang Y, Zhang H (2013) Graphene-based electrochemical sensors. Small 9:1160–1172
Zhou W, Li W, Xie Y, Wang L, Pan K, Tian G, Li M, Wang G, Qu Y, Fu H (2014) Fabrication of noncovalently functionalized brick-like β-cyclodextrins/graphene composite dispersions with favorable stability. RSC Adv 4:2813–2819
Mathapa BG, Paunov VN (2013) Cyclodextrin stabilised emulsions and cyclodextrinosomes. Phys Chem Chem Phys 15:17903–17914
Karuppiah C, Palanisamy S, Chen SM, Veeramani V, Periakaruppan P (2014) Direct electrochemistry of glucose oxidase and sensing glucose using a screen-printed carbon electrode modified with graphite nanosheets and zinc oxide nanoparticles. Microchim Acta 181:1843–1850
Palanisamy S, Sakthinathan S, Chen SM, Thirumalraj B, Wu TH, Lou BS, Liu XH (2016) Preparation of β-cyclodextrin entrapped graphite composite for sensitive detection of dopamine. Carbohydr Polym 135:267–273
Palanisamy S, Cheemalapati S, Chen SM (2012) Highly sensitive and selective hydrogen peroxide biosensor based on hemoglobin immobilized at multiwalled carbon nanotubes–zinc oxide composite electrode. Anal Biochem 429:108–115
Ren L, Dong J, Cheng X, Xu J, Hu P (2013) Hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin immobilized on gold nanoparticles in a hierarchically porous zeolite. Microchim Acta 180:1333–1340
Li P, Ding Y, Lu ZY, Li Y, Zhu XS, Zhou YM, Tang YW, Chen Y, Cai CX, Lu TH (2013) Direct electrochemistry of hemoglobin immobilized on the water-soluble phosphonate functionalized multi-walled carbon nanotubes and its application to nitric oxide biosensing. Talanta 115:228–234
Sun W, Guo Y, Ju X, Zhang Y, Wang X, Sun Z (2013) Direct electrochemistry of hemoglobin on graphene and titanium dioxide nanorods composite modified electrode and its electrocatalysis. Biosens Bioelectron 42:207–213
Palanisamy S, Karuppiah C, Chen SM, Periakaruppan P (2014) A highly sensitive and selective enzymatic biosensor based on direct electrochemistry of hemoglobin at zinc oxide nanoparticles modified activated screen printed carbon electrode. Electroanalysis 26:1984–1993
Xie L, Xu Y, Cao X (2013) Hydrogen peroxide biosensor based on hemoglobin immobilized at graphene, flower-like zinc oxide, and gold nanoparticles nanocomposite modified glassy carbon electrode. Colloids Surf B 107:245–250
Chen PY, Yang HH, Huang CC, Chen YH, Shih Y (2015) Involvement of Cu(II) in the electrocatalytic reduction of bromate on a disposable nano-copper oxide modified screen-printed carbon electrode: hair waving products as an example. Electrochim Acta 161:100–107
Zhou DD, Ding L, Cui H, An H, Zhai JP, Li Q (2012) Fabrication of high dispersion Pd/MWNTs nanocomposite and its electrocatalytic performance for bromate determination. Chem Eng J 200–202:32–38
Salimia A, MamKhezri H, Hallaj R, Zandi S (2007) Modification of glassy carbon electrode with multi-walled carbon nanotubes and iron(III)-porphyrin film: application to chlorate, bromate and iodate detection. Electrochim Acta 52:6097–6105
Thangamuthu R, Wu YC, Chen SM (2009) Silicomolybdate-incorporated-glutaraldehyde- cross-linked poly-L-lysine film modified glassy carbon electrode as amperometric sensor for bromate determination. Electroanalysis 21:1655–1658
Marafon E, Kubota LT, Gushikem Y (2009) FAD-modified SiO2/ZrO2/C ceramic electrode for electrocatalytic reduction of bromate and iodate. J Solid State Electrochem 13:377–383
Acknowledgments
This project was supported by the National Science Council and the Ministry of Education of Taiwan (Republic of China). The financial supports of this work by the Ministry of Science and Technology (MOST), Taiwan (MOST-104-2410-H-182-015 to BSL and NSC101-2113-M-027-001-MY3 to SMC) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 905 kb)
Rights and permissions
About this article
Cite this article
Palanisamy, S., Wang, YT., Chen, SM. et al. Direct electrochemistry of immobilized hemoglobin and sensing of bromate at a glassy carbon electrode modified with graphene and β-cyclodextrin. Microchim Acta 183, 1953–1961 (2016). https://doi.org/10.1007/s00604-016-1811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1811-x