Abstract
Magnetite (Fe3O4) nanoparticles were coated with tannic acid to give nanoparticles (NPs) of the type Fe3O4@TA and are shown to be a viable sorbent for preconcentration of Cd2+, Co2+ and Cr3+. The size, morphology, composition, and properties of the Fe3O4@TA NPs were characterized by field emission scanning electron microscopy, energy-dispersive X-ray analysis, vibrating sample magnetometery and FTIR. They were applied to the solid-phase extraction of the metal ions from environmental water samples prior to their quantitation by flow injection inductively coupled plasma-optical emission spectrometry. The effects of sample solution, extraction and desorption times, kind of eluent and quantity of sorbent were optimized. The calibration plots are linear in the concentration ranges from 0.5 to 100 μg L−1 (for both Cd and Co) and from 0.2 to 100 μg L−1 (for Cr). The limits of detection are between 0.1 and 0.2 μg L−1. The intra-day relative standard deviations based on four replicates are in the range of 6.1 to 7.1 %. The method was successfully applied to the determination of the three metal ions in (spiked) tap water, mineral water, and river water. Recoveries varied in the range from 90 to 109 %, this confirming the good performance of the method.

Tannic acid-coated Fe3O4 nanoparticles (TA@ Fe3O4 NPs) have been successfully synthesized. The NPs were applied as an efficient sorbent for extraction and pre-concentration of Cd2+, Co2+ and Cr3+ from environmental water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are among the main sources of pollution in the environment [1]. So, determination of metal ions at trace levels in the environmental samples is essential. Various instrumental techniques such as spectrophotometry, electrothermal atomic absorption spectrometry (ETAAS) [2], inductively coupled plasma-optical emission spectrometry (ICP-OES) [3], inductively coupled plasma-mass spectrometry (ICP-MS) [4], and flame atomic absorption spectrometry (FAAS) [5] are widely applied for determination of heavy metal ions. In these instrumental determinations, low concentrations of analytes and complexity of matrices are the main challenges [6]. These limitations may be overcome by applying a separation–preconcentration procedure such as solvent extraction [7], co-precipitation [8], cloud point extraction (CPE) [9], ion exchange [10] or solid phase extraction (SPE) [11–14] before instrumental analysis of metal ions. Among the sample preparation methods, SPE is one of the most effective preconcentration techniques due to its simplicity, high extraction efficiency, and low consumption of organic solvents [15].
Recently, magnetic nanoparticles (MNPs) have attracted substantial interest in various scientific fields because of their special properties such as their super paramagnetic properties, high dispersibility, low toxicity and easiness of their surface modification [16, 17]. These materials have two main applications in analytical chemistry; separation or purification of chemical species (mainly magnetic solid-phase extraction (MSPE)) and in sensors or biosensors [18].
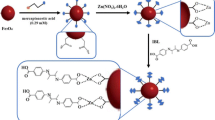
Moreover, MNPs are considered as adsorbents that have attracted many researchers to SPE methods substantially because of their unique properties such as excellent magnetic responsivity, significantly higher surface area-to-volume ratio, and short diffusion route, which lead to high extraction capacity, rapid extraction dynamics, and high extraction efficiencies [19]. In addition, functionalized MNPs as solid-phase extracting agents have increasingly attracted great attention. More recently, different types of functional groups have been evaluated to provide suitable MNPs for separation and preconcentration of various analytes from different samples. Among these surface modified nanoparticles, magnetic Fe3O4 nanoparticles modified with C8 [20], C18 [21, 22], n-octadecylphosphonic acid [23], multi-walled carbon nanotube [24], γ-mercaptopropyltrimethoxysilane [25], and 2-amino-5-mercapto-1,3,4-thiadiazole [26] have been introduced for extraction and preconcentration of organic pollutants and metal ions. One of the common modifiers is humic substances containing carboxyl and hydroxyl functional groups well known as metal scavengers. It was reported that the surface properties and thus the adsorption capacities of hydrous ferric oxide remarkably enhanced using humic substances [27]. In this study, commercially available tannic acid (TA) is employed as a humic-like substance in the modification of MNP to improve the surface properties and adsorption capacity of the adsorbent for toxic metal ions. TA is a natural organic polyphenol containing sugar esters mainly derived from breakdown of the herbs. It possesses a lot of phenolic hydroxyl and carbonyl functional groups on the framework [28]. The precipitation and complexation of polyphenols with polyvalent cations in simple aqueous solutions is well known from the work of earlier investigators [29]. Therefore, the nanoadsorbent (Fe3O4@TA NPs) has potential applications for removal and recovery of toxic metal ions from aqueous systems.
The aim of this study was to develop a NP-based SPE method for preconcentration and determination of trace amounts of some heavy metal ions including Cd2+, Co2+, and Cr3+ from environmental water samples. To the best of our knowledge, Fe3O4@TA NPs have not been employed previously for the extraction of the studied toxic metal ions in aqueous samples.
Experimental
Reagents and materials
All chemicals used were of analytical reagent grade. Stock solutions (1000 mg L−1) of Cd2+, Co2+, and Cr3+ were prepared by direct dissolution of proper amounts of Cd(NO3)2.4H2O, Co(NO3)2.6H2O, and Cr(NO3)3·9H2O salts from Merck (Darmstadt, Germany, http://www.merckgroup.com) in ultra-pure water. Other reagents including ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), ammonium hydroxide (25 %), nitric acid, and TA were purchased from Merck. The standard solutions were diluted with ultra-pure water to prepare mixed standards. The pH of solutions was adjusted by dropwise addition of NaOH (0.5 mol L−1) and HNO3 (0.5 mol L−1). The ultra-pure water used was purified on AquaMax–Ultra Youngling Ultra-pure water purification system (Dongan-gu, South Korea, wk101406080.company.weiku.com).
Apparatus
The particle size and morphology of the modified NPs were determined by a field emission scanning electron microscope model Zeiss Sigma-VP FESEM instrument (Jena, Germany, http://www.zeiss.com). Chemical composition of the synthesized NPs was analyzed using energy-dispersive X-ray spectroscopy (EDX) by applying Zeiss Sigma-VP FESEM instrument (Jena, Germany). Surface modification of Fe3O4 NPs was investigated by Fourier-transform infrared (FT-IR) spectroscopy from Thermo scientific Nicolet IR100 FT-IR spectrometer (Madison, WI, USA, http://www.coleparmer.com) in the frequency range of 570–4000 cm−1 by pelletizing the homogenized powder of synthesized NPs and KBr. Magnetic property was measured by a vibrating sample magnetometer (VSM) (AGFM/VSM 3886 Kashan, Iran) at room temperature in a magnetic field strength of 1 Tesla.
A radial view Varian Vista-Pro simultaneous ICP-OES (Springvale, Australia, www.Varianinc.com) equipped with a charge-coupled device (CCD) was applied for determination of the metal ions. A homemade sample loop with internal volume of 250 μL prepared from silicon tube was used for flow injection introduction of the preconcentrated phase into the nebulizer of ICP-OES. The pH values of the solutions were measured by a WTW pH meter from Inolab (Weilheim, Germany; www.WTW.com) equipped with a combined glass electrode.
Preparation of tannic acid-coated magnetic nanoparticles (Fe3O4@TA NPs)
Fe3O4 NPs were prepared by chemical co-precipitation method. Briefly, FeCl3·6H2O (8.48 g) and FeCl2·4H2O (2.25 g) were dissolved in 400 mL deionized water under nitrogen atmosphere with vigorous stirring (1000 rpm) at 80 °C for 20 min. Then, 20 mL ammonia solution (25 % wt) was added to the solution. The color of bulk solution immediately changed from orange into black. After stirring the mixture for 2 min, an aqueous solution containing 3 g TA was added to the solution. Afterwards, the solution was stirred for 3 min, and in the following the Fe3O4@TA NPs precipitate obtained was separated by magnetic decantation and washed several times with deionized water. Finally, the Fe3O4@TA NPs obtained were dried in a vacuum oven at 70 °C for 5 h.
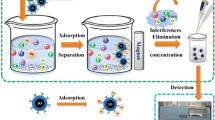
Extraction procedure
First, 50 mg of Fe3O4@TA NPs was added to 100 mL water containing the given amount of target analytes in a 250-mL beaker and the pH value was adjusted to 6. NPs were in colloidal state for 10 min and then settled down. After mechanical stirring (400 rpm) of the mixture for 10 min, the sorbent was immediately separated from the suspension with a magnet (10 × 5 × 4 cm with a 1.4 Tesla magnetic field) and the supernatant was decanted. Finally, the preconcentrated target analytes were desorbed from the sorbent with 400 μL of 0.2 mol L−1 HNO3 solution by fierce vortex for 2 min. Then, the final solution was injected into the ICP instrument using a six-way two-position injection valve.
Results and discussion
Choice of materials
The nm-sized metal oxides are not target selective and are unsuitable for samples with complex matrices [18]. Therefore, a suitable coating is essential to overcome such limitations. Also, surface modification stabilizes the NPs and also prevents their oxidation. However, MNPs can efficiently be functionalized based on the formation of relatively stable linker between hydroxyl groups on the NPs surface and suitable anchoring agents such as phosphonic acid and dopamine derivatives [30]. So, TA as a humic-like substance having a lot of phenolic hydroxyl and carbonyl functional groups on the framework can be used in the modification of MNP (Fig. 1). Moreover, the feasibility of complexation of polyphenols with polyvalent cations in simple aqueous solutions can improve the surface properties and adsorption capacity of the Fe3O4@TA NPs for toxic metal ions. Therefore, the novel nanoadsorbent (Fe3O4@TA NPs) has potential ability for removal and recovery of toxic metal ions from aqueous systems.
Characterization of Fe3O4@TA nanoparticles
Field emission scanning electron microscopy observations (FESEM) were performed to characterize the morphology and size of the synthesized Fe3O4@TA NPs. Figure 2 depicts FESEM images. They show that Fe3O4@TA NPs exhibit near spherical morphology with a size distribution in the range of 30 ± 8 nm for 52 particles.
The atomic composition of the Fe3O4@TA NPs was evaluated by EDX analysis. A typical EDX spectrum taken from the NPs is presented in Fig. S1 (Electronic Supplementary Material), where peaks associated with Fe, C, and O can be distinguished. The quantitative analysis gives weight ratios of Fe (58.7 %), C (19.9 %), and O (19.0 %).The above results are consistent with the results obtained from FT-IR and provide clear evidence for TA coating onto the Fe3O4 NPs.
FT-IR spectral analysis disclosed the surface chemistry of the bare and coated Fe3O4 NPs. In the spectra of pure Fe3O4 NPs and Fe3O4@TA NPs (Fig. S2 (a) and (b)), the broad band at 3446 cm−1 can be assigned to stretching of O-H bond of Fe3O4 NPs, while one characteristic peak can be observed at 589 cm−1, which corresponds to the Fe-O stretching mode of the Fe3O4 lattice. There are several additional peaks in the spectrum of Fe3O4@TA NPs belonging to the TA coating (Fig. S2(b)). Absorption bands at 1695 and 1072 cm−1 belong to stretching vibrations of C = O and C-O, respectively. According to these results, FT-IR analyses suggest that TA is successfully coated on the surface of Fe3O4 NPs.
The magnetic properties of the NPs were characterized by the VSM and Fig. 3 shows the plots of room temperature magnetization (M) versus magnetic field (H) (M-H curves or hysteresis loops) of Fe3O4 NPs (a) and Fe3O4@ TA NPs (b). Determination of the saturation magnetization (Ms), remanent magnetization (Mr) and coercivity (Hc) is possible by the hysteresis curve [17, 31]. The magnetization of samples is saturated at high fields of up to ±7500.0 Oe and the Ms. of samples changed from 56.8 to 27.4 emu g−1, due to the coating of TA around the Fe3O4 core. The hysteresis loops show the super-paramagnetic behavior of the Fe3O4@TA NPs in which the Mr. and the Hc are close to zero (Mr = 0.19 emu per g, and Hc = 2.70 Oe, respectively).
Optimization of conditions for magnetic solid phase extraction (MSPE)
A mixture solution containing 100 μg L−1 of each heavy metal ion was used in optimization experiments. All quantifications were done based on the peak height. The following parameters were optimized to obtain the highest extraction efficiency of metal ions using Fe3O4@TA NPs sorbent: (a) Sample pH value: The effect of pH on efficiency of extraction of the analytes on the adsorbent surface was studied by varying the pH in the range of 4 to 10. The results demonstrated that the maximum adsorption efficiency was achieved at pH 6 for target metal ions (Fig. S3). At higher pH values, decrease in the extraction efficiency may be described by formation and precipitation of hydroxide species of metal ions. Under strong acidic conditions, decrease in the extraction efficiency was probably due to the surface protonation of the adsorbent and occupation of active sites by protons rather than target metal ions. Hence, pH 6 was selected as the optimal value in subsequent experiments; (b) Concentration and volume of the eluent: In order to optimize the concentration and volume of eluent, various concentrations of HNO3 solutions (0.001–0.5 mol L−1) were used to desorb the adsorbed analytes. As shown in Fig. S4, 0.2 mol L−1 HNO3 is enough to elute the metal ions. Higher concentrations of HNO3 can relatively decompose the NPs. To achieve the highest enrichment and better recovery of the adsorbed analytes, the effect of the eluent volume was also tested. The experimental results indicated that 400 μL of 0.2 mol L−1 HNO3 is enough to obtain satisfactory recoveries and enrichment of metal ions. Therefore, 400 μL of 0.2 mol L−1 HNO3 was selected to ensure complete elution of analytes for further experiments; (c) Adsorbent amount: To find the optimal amount of sorbent to extract 100 μg L−1 of the metal ions from 100 mL of the solution, different amounts of adsorbent in the range of 20–80 mg was added to the solution (Fig. S5). Recovery of metal ions is quantitative when using 50 mg of sorbent. By increasing the adsorbent amount, surface area and accessible sites of the sorbent increase; so extraction efficiency increases, too. However, the adsorption efficiency was decreased with a further increase in the adsorbent amount. By using a fixed volume of the eluent, elution of the analyte is not complete from large amounts of adsorbent. Accordingly, 50 mg of the sorbent was used in further experiments; (d) Effect of adsorption and desorption times: The extraction times were varied in the range of 2–20 min. At the beginning of the process, recoveries increased rapidly by increasing extraction time from 2 to 10 min. After this time, no substantial increase was obtained with longer extraction time. So, based on this result, 10 min was chosen as the optimal adsorption time. The effect of desorption time on extraction efficiency of the metal ions were evaluated in the range of 1-5 min. The experimental results indicated that 2 min is sufficient for quantitative desorption of heavy metal ions by 400 μL of 0.2 mol L−1 HNO3. According to data, the following experimental conditions were found to give best results: (a) A sample pH value of 6; (b) a reaction/extraction time of 10 min; (c) desorption time of 2 min; (d) concentration and volume of the eluent of 0.2 mol L−1 and 400 μL, respectively; and (e) adsorbent amount of 50 mg.
Reusability studies
To investigate the ability of adsorbent to be regenerated and reused, several (adsorption/desorption) regeneration cycles were carried out for the adsorbent. After each extraction process, the adsorbent was rinsed with 1 mL of eluent (0.03 mol L−1 HNO3) four times and then used in the subsequent extractions. It was found that the recoveries of the ions are diminished after two successive extraction processes.
Interference studies
The effect of some potentially interfering ions, which may interfere with the method or/and often accompany analyte ions in various real samples, was examined. Effect of potentially interfering ions including: Li+, K+, Na+, Ca2+, Mg2+, Zn2+, Pb2+, Fe2+, Mn2+, Ni2+, and Cu2+ on preconcentration/separation and determination of the target heavy metal ions (at 100 μg L−1 level of each heavy metal ion) was examined under optimal conditions. The interferences studies were performed by adding each ion separately. The results given in Table 1 demonstrate that the presence of diverse potentially interfering ions has no obvious influence on determination of Cd2+, Co2+, and Cr3+under the conditions selected, indicating that the present adsorbent has a potential to be applied to analyze real samples.
Method performance
Under the optimal extraction conditions, the figures of merit of the proposed technique in terms of enhancement factor (EF), limit of detection (LOD), linear dynamic range (LDR), and repeatability were evaluated. Performance of the developed procedure is summarized in Table 2. The calibration curves were constructed using 10 spiking levels of analytes. For each level, four replicate experiments were performed. The linearity was observed in the concentration range from 0.2 to 100 μg L−1 for Cr and 0.5 to 100 μg L−1 for Co and Cd, with the correlation of determinations (R2) ranging from 0.998 to 0.999. The LODs, based on a signal-to-noise ratio (S/N) of 3, were determined to be 0.1 μg L−1 for Cr and 0.2 μg L−1 for Co and Cd. Intraday precisions were obtained from four consecutive replicates and expressed as relative standard deviations (RSDs%). The values were between 6.1 and 7.1 %. Under optimal conditions, the preconcentration factors for Cd2+, Co2+, and Cr3+ were obtained as 113, 118, and 90, respectively.
A comparison between the figures of merit of the proposed method and some of the published methods for extraction and determination of Cd2+, Co2+, and Cr3+ are summarized in Table 3. Clearly, the linearity, limit of detection, and precision of the method are comparable to or better than the results of other methods reported for extraction and determination of the target analytes. Furthermore, there is possibility of extraction of analytes from larger sample volumes at shorter extraction times and no need for centrifugation and filtration step in comparison with conventional SPE sorbents. Also, the method does not need a chelating agent or organic solvent. All the results reveal that the new developed method is a good sample preconcentration technique that can be used for ultra-trace analysis of the target analytes in real samples.
Analysis of real samples
To demonstrate the applicability and reliability of the present method for real samples, it was successfully applied to determination of the metal ions in some real samples including tap water from Tarbiat Modares University (Tehran, Iran), mineral, and river waters. The accuracy of the method was verified by calculating the relative recovery studies in the real samples. Under the optimized conditions, 100 mL of each sample was extracted by the TA@Fe3O4 NPs. The results are shown in Table 4. It can be seen that relative recoveries of the metal ions (expressed as the mean percentage between the amounts found and the ones spiked) in the real water samples were in the range of 90–109 %, which implies that the matrix have negligible effect on efficiency of the proposed method.
Conclusions
In the present study, a simple, sensitive and reliable MSPE-FI-ICP-OES method was developed using TA-coated Fe3O4 nanoparticles as a magnetic solid phase extraction sorbent for preconcentration of Cd2+, Co2+, and Cr3+ ions in aqueous solutions. Fe3O4 NPs were successfully coated with TA and characterized by SEM, EDX, VSM and FT-IR. The present MSPE method have advantages such as (a) no consumption of chelating agent and organic solvents that is commonly used in complexation and extraction of metal ions, (b) being less time-consuming due to the high specific surface area, absence of internal diffusion resistance, operation in dispersion mode, and magnetic separation, and (c) easiness of synthesis of modified MNPs in the laboratory. So, with the characteristics of enhanced efficiency, good recoveries and precision, wide linearity, and great enrichment factor, MSPE-FI-ICP-OES method would provide an exciting low-cost and environmental friendly method for simple and sensitive determination of Cd2+, Co2+, and Cr3+ ions in various real water samples.
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407. doi:10.1016/j.jenvman.2010.11.011
Manzoori JL, Abdolmohammad-Zadeh H, Amjadi M (2007) Simplified cloud point extraction for the preconcentration of ultra-trace amounts of gold prior to determination by electrothermal atomic absorption spectrometry. Microchim Acta 159:71. doi:10.1007/s00604-0727-2
Cui Y, Chang X, Zhu X, Luo H, Hu Z, Zou X, He Q (2007) Chemically modified silica gel with p-dimethylaminobenzaldehyde for selective solid-phase extraction and preconcentration of Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by ICP-OES. Microchem J 87:20. doi:10.1016/j.microc.2007.04.004
Li J, Zhong L-F, Tu X-L, Liang X-R, Xu J-F (2010) Determination of rhenium content in molybdenite by ICP–MS after separation of the major matrix by solvent extraction with N-benzoyl-N-phenylhydroxalamine. Talanta 81:954. doi:10.1016/j.talanta.2010.01.043
Praveen RS, Daniel S, Prasada Rao T (2005) Solid phase extraction preconcentration of cobalt and nickel with 5,7-dichloroquinone-8-ol embedded styrene–ethylene glycol dimethacrylate polymer particles and determination by flame atomic absorption spectrometry (FAAS). Talanta 66:513. doi:10.1016/j.talanta.2004.11.026
Yamini Y, Faraji M, Adeli M (2015) Magnetic silica nanomaterials for solid-phase extraction combined with dispersive liquid-liquid microextraction of ultra-trace quantities of plasticizers. Microchim Acta 182:1491. doi:10.1007/s00604-015-1474-z
Banda R, Jeon H, Lee M (2012) Solvent extraction separation of Pr and Nd from chloride solution containing La using cyanex 272 and its mixture with other extractants. Sep Purif Technol 98:481. doi:10.1016/j.seppur.2012.08.015
NumanBulut V, Duran C, Gundogdu A, Soylak M, Yildirim N, Elci L (2008) A new approach to separation and pre-concentration of some trace metals with co-precipitation method using a triazole. Talanta 76:469. doi:10.1016/j.talanta.2008.03.040
Ojeda CB, Rojas FS (2012) Separation and preconcentration by cloud point extraction procedures for determination of ions: recent trends and applications. Microchim Acta1 77:1. doi:10.1007/s00604-011-0717-x
Badawy NA, El-Baya AA, Abdel-Aal AY, Garamon SE (2009) Chromatographic separations and recovery of lead ions from a synthetic binary mixtures of some heavy metal using cation exchange resin. J Hazard Mater 166:1266. doi:10.1016/j.jhazmat.2008.12.044
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172. doi:10.1016/j.aca.2009.11.053
Cui Y, Liu S, Wei K, Liu Y, Hu Z (2015) Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim Acta 182:1337-. doi:10.1016/j.cej.2013.12.067
Soylak M, Ercan O (2009) Selective separation and preconcentration of copper (II) in environmental samples by the solid phase extraction on multi-walled carbon nanotubes. J Hazard Mater 168:1527. doi:10.1016/j.jhazmat.2009.03.032
Samadi A, Amjadi M (2015) Magnetic Fe3O4@C nanoparticles modified with 1-(2-thiazolylazo)-2-naphthol as a novel solid-phase extraction sorbent for preconcentration of copper (II) Volume. Microchim Acta182:257. doi:10.1016/j.jhazmat.2012.04.001
Zang Z, Li Z, Zhang L, Li R, Hu Z, Chang X, Cui Y (2010) Chemically modified attapulgite with asparagine for selective solid-phase extraction and preconcentration of Fe(III) from environmental samples. Anal Chim Acta 663:213. doi:10.1016/j.aca.2010.01.057
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: synthesis and application as a new sorbent for solid-phase extraction. Anal Chim Acta 770:68. doi:10.1016/j.aca.2013.01.043
Moghanian H, Mobinikhaledi A, Blackman AG, Sarough-Farahani E (2014) Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amido- and 1-aminoalkyl-2-naphthols. RSC Adv 4:28176. doi:10.1039/c4ra03676j
Aguilar-Arteaga K, Rodriguez JA, Barrado E (2010) Magnetic solids in analytical chemistry: a review. Anal Chim Acta 674:157. doi:10.1016/j.aca.2010.06.043
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A Review Anal Chim Acta 789:1. doi:10.1016/j.chroma.2008.01.047b
Yao N, Chen H, Lin H, Deng C, Zhang X (2008) Enrichment of peptides in serum by C8-functionalized magnetic nanoparticles for direct matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. J Chromatogr A 1185:93. doi:10.1016/j.chroma.2008.01.047
Jiang C, Sun Y, Yu X, Gao Y, Zhang L, Wang Y, Zhang H, Song D (2014) Application of C18-functional magnetic nanoparticles for extraction of aromatic amines from human urine. J Chromatogr B 947–948:49. doi:10.1016/j.jchromb.2013.12.008
Zhang S, Niu H, Cai Y, Shi Y (2010) Barium alginate caged Fe3O4-C18 magnetic nanoparticles for the pre-concentration of polycyclic aromatic hydrocarbons and phthalate esters from environmental water samples. Anal Chim Acta 665:167. doi:10.1016/j.aca.2010.03.026
Ding J, Gao Q, Luo D, Shi Z-G, Feng Y-Q (2010) n-Octadecylphosphonic acid grafted mesoporous magnetic nanoparticle: Preparation, characterization, and application in magnetic solid-phase extraction. J Chromatogr A 1217:7351. doi:10.1016/j.chroma.2010.09.074
Luo C, Tian Z, Yang B, Zhang L, Yan S (2013) Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem Eng J 234:256. doi:10.1016/j.cej.2013.08.084
Huang C, Hu B (2008) Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim Acta B 63:437. doi:10.1016/j.sab.2007.12.010
Mashhadizadeh MH, Karami Z (2011) Solid phase extraction of trace amounts of Ag, Cd, Cu, and Zn in environmental samples using magnetic nanoparticles coated by 3-(trimethoxysilyl)-1-propantiol and modified with 2-amino-5-mercapto-1,3,4-thiadiazole and their determination by ICP-OES. J Hazard Mater 190:1023. doi:10.1016/j.jhazmat.2011.04.051
Zhang X, Zhang P, Wu Z, Zhang L, Zeng G, Zhou C (2013) Adsorption of methylene blue onto humic acid-coated Fe3O4 nanoparticles. Colloid Surf A 435:85. doi:10.1016/j.colsurfa.2012.12.056
Üçer A, Uyanik A, Aygün ŞF (2006) Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Sep Purif Technol 47:113. doi:10.1016/j.seppur.2005.06.012
Slabbert N (1992) Complexation of condensed tannins with metal ions. In: Hemingway RW, Laks PE (eds) Plant polyphenols. Plenum Press, New York, pp. 421–436
Mazur M, Barras A, Kuncser V, Galatanu A, Zaitzev V, Turcheniuk KV, Woisel P, Lyskawa J, Laure W, Siriwardena A, Boukherroub R, Szunerits S (2013) Iron oxide magnetic nanoparticles with versatile surface functions based on dopamine anchors. Nanoscale 5:2692. doi:10.1039/c3nr33506b
Moghanian H, Mobinikhaledi A, Baharangiz Z (2014) Synthesis, characterization and magnetic properties of novel heat resistant polyimide nanocomposites derived from 14 H-dibenzo [a,j] xanthenes. J Polym Res 21:513. doi:10.1007/s10965-014-0513-5
Tuzen M, Soylak M (2009) Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. J Hazard Mater 164:1428. doi:10.1016/j.jhazmat.2008.09.050
Yamini Y, Faraji M, Shariati S, Hassani R, Ghambarian M (2008) On-line metals preconcentration and simultaneous determination using cloud point extraction and inductively coupled plasma optical emission spectrometry in water samples. Anal Chim Acta 612:144. doi:10.1016/j.aca.2008.02.034
Tuzen M, Soylak M, Elci L (2005) Multi-element pre-concentration of heavy metal ions by solid phase extraction on Chromosorb108. Anal Chim Acta 548:101
Ngeontae W, Aeungmaitrepirom W, Tuntulani T (2007) Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II) and Cd(II). Talanta 71:1075. doi:10.1016/j.talanta.2006.05.094
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 90.9 kb)
Rights and permissions
About this article
Cite this article
Bagtash, M., Yamini, Y., Tahmasebi, E. et al. Magnetite nanoparticles coated with tannic acid as a viable sorbent for solid-phase extraction of Cd2+, Co2+ and Cr3+ . Microchim Acta 183, 449–456 (2016). https://doi.org/10.1007/s00604-015-1667-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1667-5