Abstract
Gold nanoclusters (AuNCs) stabilized with bovine serum albumin were utilized as a fluorescent probe for ferrous ion. The detection scheme is based on the quenching of the fluorescence of the modified AuNCs by hydroxyl radical (•OH) that is generated in the Fenton reaction between Fe(II) and H2O2. Fe(II) can be quantified in the 0.08 to 100 μM concentration range, and the limit of detection is as low as 24 nM. The method also displays good accuracy and high sensitivity when employed to the determination of Fe(II) in rat cerebrospinal fluids (CSFs). When applied to CSFs of a rat model of Alzheimer’s disease, it revealed enhanced levels of Fe(II) compared to a control, thereby showing the important physiological role of iron(II) in this disease.

BSA-stabilized gold nanoclusters (BSA-AuNCs) were utilized for the determination of ferrous ion in rat cerebrospinal fluids. The method is based on the quenching of the fluorescence by hydroxyl radical (•OH) which is generated in the Fenton reaction between Fe(II) and H2O2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is the most affluent metal ion in cellular systems and of outstanding significance owing to its essential roles in biological systems [1]. Almost all cells employ iron ions as a cofactor for fundamental biochemical activities, such as oxygen transport, energy metabolism and DNA synthesis, based upon its ability to switch between the two most common redox states: ferrous, Fe(II) and ferric, Fe(III) [2]. Disruptions in iron homeostasis from both iron deficiency and overload account for some of the most common human diseases, such as anemia, Parkinson’s syndrome, Alzheimer’s disease and cancer [3]. Determination of iron content in biological systems is thus helpful for the diagnosis of some relevant diseases. However, much effort have been focused on the more stable and prevalent oxidation state, Fe3+, and various probes specific for it have been reported [4, 5]. On the other hand, powerful method for detection of chemically more reactive species, Fe2+, is rather limited.
The emergence of new kinds of fluorescent nanomaterials such as gold nanoclusters (AuNCs) is now generating new opportunities for targeting applications [6]. AuNCs [7] typically consist of several tens to a hundred of gold atoms, with small size regime comparable to Fermi wavelength of the conduction electrons. Since the continuous density of states breaks into discrete energy levels, AuNCs exhibit molecule-like properties in absorption and fluorescence [8]. Particularly, protein-templated AuNCs have attracted special attention due to their facile synthesis, strong fluorescence emission, high photostability, non-toxicity and high biocompatibility [9]. They have been studied widely, and free bilirubin [10] in blood serum samples and pathogenic bacteria [11] were detected using human serum albumin encapsulated gold nanoclusters (HSA-AuNCs). Some other biologically important molecules such as cystatin C [12], biothiols [13], glucose [14], cysteine [15], xanthine [16], acetylcholinesterase activity [17] etc. have been successfully determined with bovine serum albumin-stabilized gold nanoclusters (BSA-AuNCs). Cyanide [18] and mercury ion [19] have also been determined based on the quenching of BSA-AuNC fluorescence.
In an investigation aimed at the development of a convenient and cost-effective procedure for assay of Fe2+ in biological and related circumstances, we established a fluorometric method with biocompatible BSA-AuNCs for the determination of ferrous ion. It is proven that the fluorescence of BSA-AuNCs is quenched by hydroxyl radical (•OH) [20], which is produced in the Fenton reaction between Fe2+ and H2O2. Then Fe2+ can be determined by the amount of generated •OH through fluorescent quenching. The method is sensitive, simple and rapid, and has demonstrated its feasibility by detecting ferrous ion in cerebrospinal fluids (CSFs). Due to the importance of iron in inflammation and oxidative stress that occurs with aging and, particularly, in Alzheimer’s disease (AD) [21], we selected rat model of AD and determined ferrous ion in CSFs. The results showed enhanced CSFs ferrous ion content compared with control, which proved marked disease-associated changes in the iron content of the AD brain.
Experimental
Chemicals and reagents
Bovine serum albumin (BSA) and ascorbate oxidase (AO) were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com). Chloroauric acid (HAuCl4 · 4H2O), ferrous chloride (FeCl2), sodium acetate trihydrate (CH3COONa · 3H2O), acetic acid (CH3COOH), ascorbic acid (AA) and hydrogen peroxide (H2O2) were purchased from Sinopharm Chemical Reagent Co. Ltd. (http://www.sinoreagent.com). The stock solution of H2O2 was freshly diluted from 30 % solution. All other chemicals, such as ferric chloride (FeCl3), were of analytical grade and used without further purification. All solutions were prepared with water purified by a Milli-Q Purification System (http://www.merckmillipore.com/CN/en).
Synthesis and characterization of BSA-AuNCs
BSA-stabilized gold nanoclusters were synthesized in aqueous solution according to a previous publication [22]. In a typical experiment, all glassware used in the experiments was cleaned in a bath of freshly prepared aqua regia (HCl : HNO3 = 3 : 1, V : V), and rinsed thoroughly with purified water prior to use. 15.0 mL aqueous HAuCl4 solution (10 mM) was added to BSA solution (15.0 mL, 50 mg · mL−1) under vigorous stirring at 37 °C. Two minutes later, 1.5 mL of 1 M NaOH solution was introduced and the mixture was allowed to incubate at 37 °C under vigorous stirring for 24 h. The color of the solution changed from light yellow to light brown, and then to deep brown. The solution was then dialyzed using a Solarbio MD44 dialysis bag (Molecular Weight Cut-off: 8000–14,000, www.shsolarbio.com) in 1 L double distilled water for 48 h to remove unreacted HAuCl4 or NaOH, and the double distilled water was changed totally every 12 h. The final solution was diluted to 200 mL with purified water and stored at 4 °C in refrigerator. The morphological characterization of BSA-AuNCs was performed by high-resolution transmission electron microscopy (HR-TEM), images were taken with a JEOL JEM2100F microscope operated at 200 kV (Japan electron optics laboratory Co., Ltd, www.jeol.co.jp/en/). As for the synthesis reproducibility of and characterization BSA-AuNCs, see Electronic Supplementary Material (ESM).
Analytical procedures
The fluorescence measurements were performed on a Perkin Elmer LS-55 spectrofluorometer (www.perkinelmer.com.cn) equipped with a quartz cell (1 cm × 1 cm) in the fluorescence mode. For fluorescence detection of Fe2+, appropriate amount of H2O2 and different concentrations of ferrous ion were added to an aliquot of 3 mL NaAc-HAc buffer (pH 5.70) solutions containing BSA-AuNCs (400 μL), which were placed in 5 mL colorimetric tubes. The mixtures were incubated at room temperature for 5 min before the spectral measurements. Finally, the fluorescence intensity of the test solution (F) and the blank solution (F0) were recorded, in which F and F0 are the maximum emission intensities of the BSA-AuNCs system in the presence and absence of Fe2+, respectively.
Sample treatment and determination
Preparation of rat cerebrospinal fluids (CSFs) samples: Two groups of Wistar female rats were used, that is, animal model group of AD and control group (ESM). Each rat was anaesthetized with 0.8 mL of 3.5 % chloral hydrate dissolved in normal saline [23], and the CSFs samples were collected using a microinjector, whose needle was connected with a plastic pipe terminated in another needle. The CSFs samples were centrifugated at 1000 rpm for 5 min, the resulting supernatants were collected for the following detection experiments.
As for the fluorescence detection of ferrous ion in biological samples, appropriate amount of H2O2 and different amount of sample solution were added to an aliquot of 3 mL NaAc-HAc buffer (pH 5.70) solutions containing BSA-AuNCs (400 μL), plus certain amount of ascorbate oxidase (AO), which were placed in 5 mL colorimetric tubes. Then fluorescence intensity was recorded using the LS-55 spectrofluorometer.
Data were expressed as the mean ± standard deviation. All experiments were repeated three times, and the data were calculated with Microsoft Excel.
Results and discussion
The fluorescent probe design
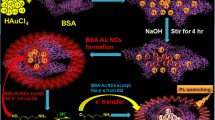
Among noble-metal nanoclusters, BSA-AuNCs are more prominent for bioanalysis due to their small size, excellent stability and biocompatibility. The working principle of fluorometric assay of Fe2+ is schematically represented in Scheme 1. BSA-AuNCs exhibit strong fluorescence, arising from intraband transitions of free electrons of the BSA-AuNCs [24]. In the presence of •OH, which is produced by Fenton reaction between Fe2+ and H2O2, the fluorescence intensity of the BSA-AuNCs can decrease significantly, attributable to the oxidation of Au(0) to Au(I) by •OH [20]. Thus it is possible for us to fabricate a facile means with BSA-AuNCs for the detection of Fe2+ ion in the presence of H2O2.
Detection of Fe2+ using BSA-AuNCs as fluorescent probe
In order to establish the proof-of-concept for our bioassay strategy outlined in Scheme 1, we conducted an experiment and evaluated the fluorescence change resulted from the interaction between BSA-AuNCs and •OH. As can be seen from Fig. 1, the emission spectrum of BSA-AuNCs (TEM image showed the average size is about 2 nm, Electronic Supplementary Material, Fig. S1) displayed an emission peak at around 638 nm upon excitation at 490 nm. Addition of Fe2+/H2O2 significantly quenched the fluorescence of BSA-AuNCs, while addition of Fe2+ or H2O2 hardly changes the fluorescence of BSA-AuNCs. The photographs of the four samples under UV light (inset, Fig. 1) also coincided with the fluorescence variation. The evolution of fluorescence intensity of the four solutions mentioned above demonstrated the feasibility of the strategy. The same effect was obtained as AuNCs decorated silica particles described in reference [20], but the synthesis of BSA-AuNCs is simpler, and no fluorescent dye is needed. Consequently, the fluorescent procedure can be established for the low-cost and simple detection of Fe2+, using biocompatible BSA-AuNCs as fluorescent probe.
The response of the fluorescent probe is usually affected by the media pH, temperature, and the reaction time. Therefore, in order to achieve sensitive detection of Fe2+, effects of these parameters were studied and optimized. pH is a crucial factor for the detection system, and we find that stability of BSA-AuNCs will change across pH range (Electronic Supplementary Material, Fig. S2) because of BSA denaturation, but the fluorescence of BSA-AuNCs remains the highest when pH ranges between 5.4 and 6.0. We then explored the effect of pH on the fluorescence difference (ΔF = F0 - F1) of BSA-AuNCs in the absence (F0) and presence (F1) of Fe2+. Correspondingly, as shown in Fig. 2a, the fluorescence intensity revealed the largest difference when pH of the media was in the range of 5.5–6.0. In weak acidic media when pH was below 5.5, ΔF increased with pH because of the decreased extent of BSA denaturation which was used for the stabilization of the gold nanoclusters; then such a difference reduced gradually when pH was above 6.0, because of both the hydrolysis of Fe2+ in neutral condition and BSA denaturation in basic media. Investigation of the effect of temperature on the fluorescence difference showed a temperature-dependent manner, as illustrated in Fig. 2b, ΔF decreased with increased temperature higher than 25 °C, owing to the decomposition of H2O2. On the other hand, the reaction was rather rapid and ΔF almost reaches the plateau only after 5 mins’ incubation (data not shown). Therefore, to get a high sensitivity for detection of Fe2+, a comparatively weak acidic media with the pH 5.7 (HAc-NaAc buffer, 0.1 M) and 5 min at room temperature were chosen for further experiments.
Analytical parameters of the method
Under the optimum experimental conditions, the capability of the fluorometric approach for the evaluation of Fe2+ was investigated. Figure 3 displayed the fluorescence spectra of BSA-AuNCs with 25 μM H2O2 in the presence of different concentrations of Fe2+. As illustrated, the fluorescence intensities decreased gradually with an increasing concentration of Fe2+. Linear ranges of Fe2+ concentrations were obtained from 0.080 to 2.5 μM and from 5.0 to 100 μM with the limit of detection (LOD) down to 24 nM (Table 1). The corresponding regression equation of the working curve, correlation coefficient (R 2), relative standard deviation of 1.0 and 20 μM Fe2+ (RSD, separately determined in parallel six times), LOD (calculated by 3S b /k, which referred to the quotient between three times of the blank reagent’s standard deviation where S b = 0.05, n = 11, and k was the slope of the working curve) and the limit of quantification (LOQ, calculated by 10Sb/k) of the fluorescent sensor were all listed in Table 1. We also tested the stability of the method, and found that BSA-AuNCs still retained its initial sensitivity toward Fe2+ after 60 days’ storage at 4 °C in refrigerator. The good long-term stability attributes to the excellent stability of the AuNCs. Accordingly, with biocompatible fluorescent probe of BSA-AuNCs, the present fluorometric method can allow for the sensitive, repeatable and stable evaluation of Fe2+.
Selectivity of the fluorescent probe for Fe2+
To evaluate the potential interference toward Fe2+ detection with BSA-AuNCs, the fluorescence response of Fe2+ (80 μM or none) together with BSA-AuNCs and 25 μM H2O2 was then investigated in the presence of competing ions or biomolecules. As shown in Fig. 4, 5000-fold excess of Na+, 3000-fold excess of K+, 500-fold excess of Ca2+, Cr3+ and Mg2+, 300-fold excess of Sn2+, Pb2+, 200-fold excess of glucose (Glu), 100-fold excess of glutathione (GSH), and 80-fold excess of cysteine (Cys), 50-fold excess of Fe3+, 6-fold excess of Mn2+, 4-fold excess of Cu2+, the signal perturbation on Fe2+ detection was generally less than ± 5.0 % (Electronic Supplementary Material, Fig. S3 showed the fluorescence spectra of BSA-AuNCs together with 25 μM H2O2 and different species). Although Mn2+ and Cu2+ may interfere with the Fe2+ determination, their content are rather low under biological circumstances (less than 10-fold of Fe2+) [25], which will not influence the accurate assay of Fe2+. Besides, it was observed that 2-fold excess of ascorbic acid (AA) would recover the fluorescence of the Fe2+ detection system, which was consistent with the results reported previously [26]. To eliminate the potential interference of AA in biological samples, a specific enzymatic reaction [27] was utilized in our experiments by the oxidation of AA to DHA with enzyme ascorbate oxidase (AO). The results demonstrate that AO effectively reduces the effect of AA for Fe2+ detection. Therefore, it is possible to use BSA-AuNCs as a fluorescent probe for Fe2+ detection in some biological samples.
Selectivity of the fluorescent sensor for Fe2+ over other representative competing ions or biomolecules in aqueous solution. Black column: fluorescence intensity of BSA-AuNCs with coexistence of 25 μM H2O2 in the absence of Fe2+; red column: in the presence of Fe2+. Concentration: Na+, 400 mM; K+, 240 mM; Ca2+, Cr3+ and Mg2+, 40 mM; Sn2+, Pb2+, 24 mM; Fe3+, 4 mM; glucose, 16 mM; GSH, 8 mM; cysteine, 6.4 mM; Mn2+, 480 μM; Cu2+, 320 μM; ascorbic acid, 160 μM; The error bars represent the standard deviation of three measurements
Determination of Fe2+ in CSFs from Wistar rats model of Alzheimer’s disease
Encouraged by the above-mentioned investigations, we evaluated if the fluorescent probe described here can be utilized to monitor Fe2+ in complex samples such as rat cerebrospinal fluids (CSFs), which is an ideal source that reflects the metabolic and pathological states of the central nervous system. It was reported that iron homeostasis in central nervous system is a very tightly regulated process [28], which is associated with aging-related progressive deterioration and with neurodegenerative disorders such as Alzheimer’s disease. We obtained Wistar female rats model of AD and determined the Fe2+ content in CSFs (Sample 1–3), normal Wistar female rats CSFs were used as control (Sample 4–6). In addition, certain amount of standard solution of Fe2+ was added into the corresponding sample solutions for testing recovery. The results were shown in Table S1. It can be seen that a good recovery of 94.5–113 % was obtained, showing the good selectivity, high accuracy of the method. What’s more, enhanced ferrous ion content in CSFs of AD model compared with control demonstrated the perturbed iron distribution and the important physiological role of iron in Alzheimer’s disease.
Comparison with other methods
Comparative information from some studies on determination of Fe(II) by various methods for the figure of merits is given in Table 2. The suggested method has relatively low LOD and wide linear range for the Fe(II) compared to other methods reported in Table 2. What’s more, our method does not need complex synthesis procedures and is more simple and cost-effective, which were favorable to the sensitive determination for Fe(II) in biological and other samples.
Conclusion
In summary, BSA-stabilized gold nanoclusters as a fluorescent probe for highly sensitive and selective determination of Fe2+ was presented. Under optimized conditions, we demonstrated the practical application of this assay by measuring the levels of Fe2+ in CSFs of Wistar female rats as AD model, indicating acceptable accuracy of the method. This strategy offers an alternative approach for low cost, simple, stable and sensitive detection of Fe2+, and is beneficial for its applications including bioassays, nanotechnology, and clinical diagnostics.
References
Hentze MW, Muckenthaler MU, Galy B, Camaschella C (2010) Two to tango: regulation of mammalian iron metabolism. Cell 142:24
Brissot P, Ropert M, Lan CL, Loréal O (2012) Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820:403
Vecchi C, Montosi G, Zhang KZ, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A (2009) ER stress controls iron metabolism through induction of hepcidin. Science 325:877
Cao HY, Chen ZH, Zheng HZ, Huang YM (2014) Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens Bioelectron 62:189
Chen YT, Jiang JZ (2013) N,N-di(2-pyridylmethyl)amino-modified porphyrinato zinc complexes. The “ON–OFF” fluorescence sensor for Fe3+. Spectrochim Acta A Mol Biomol Spectrosc 116:418
Feldheim D (2000) Nanotechnology: flipping a molecular switch. Nature 408:45
Li G, Jin RC (2014) Gold nanocluster-catalyzed semihydrogenation: a unique activation pathway for terminal alkynes. J Am Chem Soc 136:11347
Lin CA, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak W, Chang WH (2009) Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 3:395
Hofmann CM, Essner JB, Baker GA, Baker SN (2014) Protein-templated gold nanoclusters sequestered within sol–gel thin films for the selective and ratiometric luminescence recognition of Hg2+. Nanoscale 6:5425
Santhosh M, Chinnadayyala SR, Kakoti A, Goswami P (2014) Selective and sensitive detection of free bilirubin in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens Bioelectron 59:370
Chan PH, Chen YC (2012) Human serum albumin stabilized gold nanoclusters as selective luminescent probes for staphylococcus aureus and methicillin-resistant staphylococcus aureus. Anal Chem 84:8952
Lin H, Li LJ, Lei CY, Xu XH, Nie Z, Guo ML, Huang Y, Yao SZ (2013) Immune-independent and label-free fluorescent assay for Cystatin C detection based on protein-stabilized Au nanoclusters. Biosens Bioelectron 41:256
Park KS, Kim MI, Woo MA, Park HG (2013) A label-free method for detecting biological thiols based on blocking of Hg2+-quenching of fluorescent gold nanoclusters. Biosens Bioelectron 45:65
Jin LH, Shang L, Guo SJ, Fang YX, Wen D, Wang L, Yin JY, Dong SJ (2011) Biomolecule-stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens Bioelectron 26:1965
Cui ML, Liu JM, Wang XX, Lin LP, Jiao L, Zhang LH, Zheng ZY, Lin SQ (2012) Selective determination of cysteine using BSA-stabilized gold nanoclusters with red emission. Analyst 137:5346
Wang XX, Wu Q, Shan Z, Huang QM (2011) BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens Bioelectron 26:3614
Zhang N, Si YM, Sun ZZ, Li S, Li SY, Lin YH, Wang H (2014) Lab-on-a-drop: biocompatible fluorescent nanoprobes of gold nanoclusters for label-free evaluation of phosphorylation-induced inhibition of acetylcholinesterase activity towards the ultrasensitive detection of pesticide residues. Analyst 139:4620
Liu YL, Ai KL, Cheng XL, Huo LH, Lu LH (2010) Gold-nanocluster-based fluorescent sensors for highly sensitive and selective detection of cyanide in water. Adv Funct Mater 20:951
Xie JP, Zheng YG, Ying JY (2010) Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem Commun 46:961
Chen TT, Hu YH, Cen Y, Chu X, Lu Y (2013) A dual-emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species. J Am Chem Soc 135:11595
Tao Y, Wang Y, Rogers JT, Wang F (2014) Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J Alzheimers Dis 42:679
Xie JP, Zheng YG, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888
Liu DB, Chen WW, Tian Y, He S, Zheng WF, Sun JH, Wang Z, Jiang XY (2012) A highly sensitive gold-nanoparticle-based assay for acetylcholinesterase in cerebrospinal fluid of transgenic mice with Alzheimer’s disease. Adv Healthcare Mater 1:90
Zheng J, Nicovich PR, Dickson RM (2007) Highly fluorescent noble metal quantum dots. Annu Rev Phys Chem 58:409
Melo TM, Larsen C, White LR, Aasly J, Sjobakk TE, Flaten TP, Sonnewald U, Syversen T (2003) Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biol Trace Elem Res 93:1
Hu LZ, Deng L, Alsaiari S, Zhang DY, Khashab NM (2014) “Light-on” sensing of antioxidants using gold nanoclusters. Anal Chem 86:4989
Kim WS, Ye X, Rubakhin SS, Sweedler JV (2006) Measuring nitric oxide in single neurons by capillary electrophoresis with laser-induced fluorescence: use of ascorbate oxidase in diaminofluorescein measurements. Anal Chem 78:1859
Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, Palha JA, Marques F (2012) Modulation of iron metabolism in aging and in Alzheimer’s disease: relevance of the choroid plexus. Front Cell Neurosci 6:1
Li P, Fang LB, Zhou H, Zhang W, Wang X, Li N, Zhong HB, Tang B (2011) A New ratiometric fluorescent probe for detection of Fe2+ with high sensitivity and its intracellular imaging applications. Chem Eur J 17:10520
Hirayama T, Okuda K, Nagasawa H (2013) A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem Sci 4:1250
Kim H, Na YJ, Song EJ, Kyung KKB, Bae JM, Kim C (2014) A single colorimetric sensor for multiple target ions: the simultaneous detection of Fe2+ and Cu2+ in aqueous media. RSC Adv 4:22463
Praveen L, Reddy MLP, Varma RL (2010) Dans SQ can be used as turn-on fluorescent sensor for Fe2+. Tetrahedron Lett 51:6626
Acknowledgments
This work was supported by National Natural Science Foundation of China (21105057), Promotive Research Fund for Excellent Young and Middle-aged Scientist of Shandong Province (BS2013SW002) and Innovative Training Project for Undergraduate Students of Shandong Normal University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 11625 kb)
Rights and permissions
About this article
Cite this article
Yang, S., Jiang, Z., Chen, Z. et al. Bovine serum albumin-stabilized gold nanoclusters as a fluorescent probe for determination of ferrous ion in cerebrospinal fluids via the Fenton reaction. Microchim Acta 182, 1911–1916 (2015). https://doi.org/10.1007/s00604-015-1525-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1525-5