Abstract
We describe a new electrochemical platform for direct sensing of glucose. The electrode was prepared by controlled electrodeposition of nickel nanoparticles onto carbon nanotubes on a copper electrode. The sensor was optimized by investigating the effects of the concentration of nickel precursor, electrolysis time and acidity of the medium. The nanocomposite was characterized by scanning electron microscopy and energy dispersive spectroscopy. Cyclic voltammetry of glucose in 0.1 M NaOH solution gives a well-defined anodic wave with a peak potential at 0.53 V (vs. Ag/AgCl) that indicates the direct electrooxidation of glucose at the nanomaterial. The electrode responds to glucose over a wide linear range (from 2 μM to 10 mM), with high sensitivity (3.8 mA∙mM−1∙cm−2) and a low detection limit (0.7 μM). The sensor was applied to the determination of glucose in blood samples, and the results were in good agreement with data obtained by a commercially available glucometer. The method holds promise due to the ease of sensor fabrication and its robust performance and longevity.

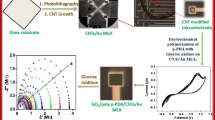
An enzyme-free glucose amperometric sensor based on nickel nanoparticle-carbon nanotubes modified copper electrode is described. The sensor is easily fabricated and shows excellent analytical performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glucose is a crucial element for life activity, which maintains the essential biochemical and physiological function. However, glycometabolism disorder usually occurs in a considerable multitude of population worldwide, resulting in clinically relevant disease such as diabetes mellitus, which commonly engenders a series of complications including cardiovascular disease, kidney failure, and blindness, and has become a major cause of death [1]. Therefore, exploring simple, rapid, reliable and cost effective methods toward glucose assay in biological specimen is highly demanded with respect to clinical diagnostic [2]. Thus far, tremendous analytical methods have been developed. Among them, electrochemical methods by immobilization of glucose oxidase (GOx) on solid electrodes represent a prevailing strategy [3], while diverse polymer films [4, 5], conducting matrix [6, 7] or redox mediators [8, 9] were usually used to bind GOx. Measurement by electroreduction of dissolved oxygen [6, 10], or by electrooxidation of hydrogen peroxide [7], an enzymatic product, have been the routine methodology toward electroanalysis of glucose. Besides, direct electrochemistry of GOx is another research topic for detection of glucose, which is generally based on the oxidation/reduction of FADH2/FAD moiety in the enzyme center [2, 11].
Enzyme electrodes have witnessed massive progress and commercially available glucose meters have also opened broad opportunities for monitoring glucose level in real time [12]; however, notable drawbacks and disadvantages have been extensively interrogated [13, 14], which originate mainly from the nature of GOx. Principally, the activity of enzyme is prone to be affected by temperature, acidity, and toxic chemicals, resulting in poor reproducibility and stability. Besides, most enzymes are expensive and thus incur high cost in usage. Additionally, laborious and complicated protocols are required to immobilize enzyme on electrode [15]. Because of these flaws aforementioned, fabrication of enzyme-free glucose sensors has continuously been motivating research interests [16].
It is well recognized that direct electrooxidation of glucose on ordinary electrodes generally encounters several challenges. For example, the analytical sensitivity is commonly restricted by the sluggish kinetics and high overpotential [12]. Moreover, the activity of metal electrodes is likely to be impaired by the adsorbed oxidation intermediates of glucose [17]. Therefore, a large number of nanomaterials have been used to enhance the electrocatalytic ability and improve antifouling property of the modified electrodes [14]. Noble metals such as gold [18], platinum [19], palladium [20], and silver [21], metal oxides [22], and bimetallic materials [15, 23, 24] have been intensively studied. These researches are bound to increase the cost of sensors, thus making design of economical glucose sensors eagerly pursued.
Nickel and copper are ideal alternatives for the fabrication of electrochemical glucose sensors [25, 26], owing to their distinctive electroactivity toward glucose as well as the abundant resource in nature. Sun [27] described a nonenzymatic glucose sensor based on copper film modified indium tin oxide (ITO) electrode, which gained a linear relationship from 1 μM to 0.5 mM. Liu [28] reported on Ni nanoparticle-loaded carbon nanofiber paste (NiCFP) electrode; the NiCFP nanocomposite was prepared by combination of electrospinning technique with thermal treatment. This method apparently relied on rather complicated electrode processing. Mu [29] presented nano nickel oxide modified electrode for nonenzymatic determination of glucose, with a detection limit of 1 μM being obtained; however, the linear response only covered the concentration range of 1–100 μM. Ni nanowire arrays were prepared by Lu [30] using a template-directed electropolymerization technique, which was used for glucose assay. The quantification capacity was up to 7 mM, and the limit of detection (LOD) was down to 0.1 μM; nevertheless this method was also carried out at the expense of sophisticated electrode fabrication. Another appealing work was demonstrated by Nie [26], where nickel nanoparticles-carbon nanotubes nanohybrid was synthesized by a precipitation reaction combined with pyroprocessing in H2/N2 mixture atmosphere, which was also somewhat inconvenient. Yang [31] et al reported chitosan-reduced graphene oxide–nickel nanoparticles decorated screen-printed electrode coupled to a microfluidic device for the determination of glucose; the LOD was 4.1 μM as regards the analysis of urine samples. Very recently, Choi [32] presented atomic layer deposition carbon nanotube–nickel nanocomposite modified glassy carbon electrode; the linear response window was from 5 μM to 2 mM, with the detection limit of 2 μM. This work although was elegant, the exceedingly complex process would make it less applicable for ordinary work.
Copper electrode can provide procedural advantages and simplification that make it more accessible for routine analysis [33]; the practical applications however, are sparse compared to other solid electrodes. We present here a simple, facile, and cost effective nonenzymatic glucose electrochemical sensor that utilized copper electrode modified with nickel nanoparticles (NiNPs)-multiwalled carbon nanotubes (MWCNTs). The sensor can be easily fabricated, thus circumventing laborious material synthesis and manufacturing procedure. In addition, the sensor showed excellent sensitivity that achieved a current density up to 3.8 mA mM−1 cm−2, and the linear range was from 2 μM to 10 mM. Furthermore, the use for glucose assay in a considerable volume of clinical samples revealed the reliability and practicability of the prepared sensor.
Materials and methods
Chemicals and reagents
All reagents were of analytical grade and used as received otherwise specified statement. Sodium phosphate monobasic, sodium hydroxide, absolute alcohol, nickel sulfate heptahydrate (guarantee reagent), were obtained from Sinopharm reagent (Shanghai, China, http://www.reagent.com.cn/). D-(+)-Glucose, ascorbic acid (AA), uric acid (UA) and urea (UE) were purchased from Aladdin (Shanghai, China, http://www.aladdinreagent.com/). MWCNTs (>95 % purity) with 5–15 μm length and 10–20 nm internal diameter were purchased from Nanotech Port (Shenzhen, China, http://www.nanotubes.com.cn/). Blood samples were kindly provided by department of laboratory medicine, the first affiliated hospital of Guangxi Medical University. 100 mM glucose stock solution was prepared in water, and diluted with supporting electrolyte to desired concentrations for analysis. Ultrapure water was prepared with a Milli-Q system (18.2 MΩ cm, MA, USA) and used throughout the experiments.
Preparation of electrochemical sensor
MWCNTs were continuously stirred in 3 M HNO3 at 60 °C for 10 h to remove the impurities, then filtered and rinsed to neutral pH value with copious amount of water, followed by drying in a vacuum oven. A portion of 5 mg of the processed MWCNTs was added into 5 ml of 0.1 % Nafion solution (in ethanol), and treated with ultrasonic agitation for 10 min. Copper disk electrode (2 mm in diameter) was polished carefully with 0.05 μm alumina slurry to a mirror finish, followed by ultrasonic cleaning in water and ethanol, each for 3 min. After drying with nitrogen, 2 μL of MWCNTs dispersion was dropped onto the surface of the electrode and allowed to dry at room temperature.
MWCNTs coated copper electrode was subjected to electrodeposition in 5 mM NiSO4 solution (0.1 M phosphate buffer, pH 5.0) at −1.2 V (vs. Ag/AgCl) for 10 min with constant nitrogen bubbling and magnetic stirring. Then prepared electrode was rinsed thoroughly with water after the electrochemical processing, and stored at 4 °C in refrigerator when not in use. Copper electrode and MWCNTs modified copper electrode were used for comparison.
Electrochemical measurement and characterization
All of electrochemical analyses were carried out with a three-electrode system, in which a platinum wire and a Ag/AgCl (Sat. KCl) electrode were used for counter electrode and reference electrode, respectively. Electrochemical measurements were performed on a CHI810 electrochemical analyzer (Shanghai, China, http://www.instrument.com.cn/) at ambient temperature. Field emission scanning electron microscopy (FESEM, Hitachi-SU8020, Japan) equipped with energy dispersive spectroscopy (EDS) was used to characterize the modified electrode.
Results and discussion
Characterization of NiNPs-MWCNTs modified Cu electrode
The nonenzymatic glucose sensor was constructed by MWCNTs decorated Cu electrode, followed by electrochemical reduction of NiSO4 to produce nickel nanoparticles. As shown in Fig. 1, Nafion-dispersed MWCNTs formed basal conducting network on Cu electrode. Upon the electrodeposition of nickel precursor in 0.1 M phosphate buffer (pH 5.0), highly uniform NiNPs adhered to the MWCNTs and assembled to form nanocluster, with a diameter of about 18 ± 2.1 nm. Such well-arranged nanoparticles ought to endow the modified electrode with more fascinating electron-transfer ability and electrocatalytic function. Additionally, EDS revealed the principal constituents (Cu, C, and Ni), while other peaks including O, Na, and P were totally attributed to the solvent used.
Electrochemical behavior of glucose
To investigate the electrochemical property of glucose on the prepared sensor, cyclic voltammetry (CV) was employed over the potential range from −0.2 to 0.8 V in 0.1 M NaOH solution. Figure 2 depicted the details. For comparison, bare Cu electrode and MWCNTs modified Cu electrode were used. Obviously, Cu electrode manifested slightly electrocatalytic activity toward glucose in alkaline environment; gradually increased oxidation current was observed in the potential region of 0.35 ∼ 0.7 V (Fig. 2a). Similar voltammetric curve was found on the MWCNTs/Cu electrode, which however, behaved fairly improved electrochemical response due to the distinct conducting power of MWCNTs (Fig. 2b). In contrast, remarkable electrolytic current of glucose emerged on the NiNPs-MWCNTs modified copper electrode (Fig. 2c), with the onset potential of 0.32 V, which then evolved to a distinct oxidation peak at 0.53 V. Such an enhanced electrocatalytic performance was exactly a result from the synergic effect of carbon nanotubes and the loaded Ni nanoparticles that feature with favorable electron transfer ability and large surface active sites. To be noted that, at the anodic process, the peak potential of the first cycle was a bit higher than that of the subsequent scans (Fig. 2c). This was also a case when blank electrolyte was subject to cyclic voltammetry. As has been reported in the literature [34], direct electrochemistry of glucose on nickel-based surface in alkaline condition commonly pertains to the following electrochemical reactions.
-
Ni + 2OH− → Ni(OH)2 + 2e
-
Ni(OH)2 + OH− → NiO(OH) + H2O + e
-
NiO(OH) + glucose → Ni(OH)2 + glucolactone
First, Ni is oxidized to form Ni2+. Then, the hydroxide phase Ni(OH)2 can be readily transferred to NiO(OH), which indeed functions as a highly-efficient catalyst and rapidly oxidizes glucose to give rise to oxidation current. Based on the above electrochemical mechanism, we can elucidate that the more positive oxidation peak in the first potential cycle differing from the succeeding scans is probably ascribed to the initial conversion from Ni to Ni2+. In the absence of glucose, a well-characteristic redox peaks can be observed, with the peak potential (E p) of 0.38 and 0.53 V, respectively; corresponding to the redox couple of Ni3+/Ni2+, which was in good agreement with the previous researches [26, 30, 31].
To achieve optimal performance of the sensor, we investigated the preparation conditions (Fig. S1, Electronic Supplementary Material, ESM). Along with the increase of electrolytic time of NiSO4, the oxidation peak current (i p) of glucose displayed an ever-increasing tendency, suggesting incremental quantity of nickel nanoparticles loaded on the electrode that directly contributed to the electrocatalytic activity. However, intensive electrolysis beyond 10 min resulted in decreased current response (Fig. S1a), mainly due to the increscent size and/or aggregation of nanoparticles, which in turn, diminished the active surface area of the sensor [24]. The effect of media acidity was also investigated (Fig. S1b); an optimal acidity was found to be pH 5. Besides, the precursor concentration was examined (Fig. S1c). Low concentration of NiSO4 (0.1 mM) generated negligible electrochemical response toward glucose, whereas pronounced augment on catalytic current was observed in the range from 0.5 to 5 mM, and then leveled off. According to these results, electrodeposition of 5 mM NiSO4 in pH 5.0 phosphate buffer for 10 min was implemented. Note that such a procedure is controllable, thus enabling the preparation of reproducible sensors and improving the practicability.
Amperometric determination of glucose
Further, cyclic voltammetry of glucose at the modified electrode with different potential scan rate (v) was tested. As shown in Fig. 3a, both anodic and cathodic peak current (i p) raised clearly with the increase of scan rate. It is calculated that i p is proportional to the square root of v, signifying a diffusion-controlled process of glucose electrooxidation on the prepared sensor. Additionally, the anodic peak shifted positively while the cathodic peak became more negative along with the scan rates, leading to much larger peak-to-peak separation.
a Serial cyclic voltammograms of glucose (1 mM in 0.1 M NaOH) on the prepared sensor at scan rate of 10, 20, 50, 100, 200, and 500 mV s−1 (from inner to outer). Inset, the relationship of anodic and cathodic peak currents versus the square root of scan rate, respectively. b Amperometric current-time curves by successive addition of glucose at bare Cu electrode, MWCNTs modified copper electrode, and the prepared sensor. Arrows indicated the concentration of glucose solutions from 2, 5, 10, 20, 50, 100, 500, 1000, 2000, 5000, 10,000 μM. Inset, i-t curve showing the electrochemical response upon addition of low concentration of glucose. Operating potential, 0.55 V (vs. Ag/AgCl)
The current-time curves by successive addition of glucose solution to 0.1 M NaOH at constant potential of 0.55 V were depicted in Fig. 3b. Consistent with the above CVs, both Cu electrode and MWCNTs/Cu electrodes generated relatively evident current response just until the introduction of 0.1 mM of glucose. In contrast, NiNPs-MWCNTs/Cu electrode produced notable current variations at very low concentration level of glucose (inset of Fig. 3b). The steady-state current can be achieved within 5 s, and responded linearly to glucose concentration, indicative of favorable electrocatalytic oxidation toward glucose. Plotting the current against the concentration yielded well-characteristic linear relationship ranging from 2 mM to 50 μM, and 0.05 to 10 mM; respectively (Fig. S2, ESM). The regression equations were correspondingly expressed as i = 0.1902C + 0.9740 (R 2 = 0.982), and i = 0.0822C + 1.770 (R 2 = 0.999). Such a broad quantification domain would be beneficial to the analytical utility. Moreover, the sensitivity was found to approach 3.8 mA mM−1 cm−2, and the limit of detection (LOD) was calculated to be 0.7 μM at signal-to-noise ratio of 3, which was advantageous to that on PtNi nanoparticle-graphene modified glassy carbon electrode [24], porous Cu foam modified screen-printed electrode [35], electrospun Ni nanoparticle-carbon nanofiber paste electrode [28], and nickel nanoparticle-chitosan-reduced graphene oxide modified screen-printed electrode [31]. This further verified the outstanding analytical performance of the prepared sensor. A comparison of representative nonenzymatic glucose sensors was listed in Table 1.
Interference and stability
The interfering electrochemical signal stemming from coexisting electroactive substances is a critical concern when fabricating a nonenzymatic glucose sensor [25]. The selectivity of the prepared sensor was examined by consecutive addition of diverse endogenous species such as ascorbic acid (AA, 0.1 mM), uric acid (UA, 0.1 mM) and urea (3 mM) by means of amperometry at the operating potential of 0.55 V. As can be seen, urea almost displays negligible current change, while AA and UA merely produce slight electrochemical response, causing 3.9 and 5.2 % signal disturbance, respectively, compared to the addition of 1 mM glucose (Fig. 4a). Considering the physiological concentration of glucose in blood (3.5 ∼ 6.1 mM) [24], such variations of current actually would not affect the detection of glucose. Interestingly, under this condition, UA led to the decline of the current terrace, which has been previously observed on a nickel-based glucose sensor [36]. The exact cause of such a phenomenon remains unclear, probably due to the electrostatic repulsion of negatively charged UA by nafion-decorated electrode surface in strong alkaline condition. Additionally, it is worthwhile to mention that dopamine has not been taken into account for the interfering test, since it has extremely low concentration level (0.01 ∼ 1 μM) in biological matrix [37], thus exclusion of potential influence.
a Electrochemical response upon alternate addition of glucose (Glu), ascorbic acid (AA), uric acid (UA), and urea (UE). Inset, the steady-state current corresponding to glucose and the potential interferents. Condition was as in Fig. 3. b Long-term stability of the sensor. The peak current originating from cyclic voltammograms was normalized (n = 3)
The stability was another important factor in view of the practicality. The experiment was performed by cyclic voltammetry of glucose in 0.1 M NaOH solution at different time interval. It can be seen that the peak current of glucose can retain 97.2 % initial value for at least 2 months (Fig. 4b), indicating a robust lifetime of the prepared sensor. This can be substantially ascribed to the rational nanostructured nickel-CNTs composite interface.
Real samples analysis
The applicability was estimated by measurement of clinical biological samples. A portion of 0.1 mL blood sample was diluted with 10-fold using blank electrolytic solution prior to electrochemical determination. The methodological validation was also carried out (Table S1, ESM). The recovery was found to be from 93.5 to 106 %, and the relative standard deviation (RSD) was less than 5.2 %, showing favorable accuracy and precision. Moreover, a blood sugar biochemical analyzer adopted in hospitals was used for reference; a total amount of 26 samples were tested (Fig. 5), and the concordant results stated clearly that the developed method was feasible for glucose assay.
Conclusion
In conclusion, we have demonstrated a novel and simple nonenzymatic glucose amperometric sensor, which is based on nickel nanoparticles/multiwalled carbon nanotubes/ copper sensing interface. The sensor can be easily fabricated at very low cost, and exhibits eminent performance with respect to sensitivity and specificity. The quantitative region spanned three orders of magnitude, and the stability was also appealing, which added much flexibility for analytical application. Furthermore, the utility was authenticated by analyzing a considerable amount of real samples; the results were in good agreement with commercial analyzer used in hospitals. This work offers great possibility for development of economical glucose electrochemical sensor.
References
Peng B, Lu J, Balijepalli AS, Major TC, Cohan BE, Meyerhoff ME (2013) Evaluation of enzyme-based tear glucose electrochemical sensors over a wide range of blood glucose concentrations. Biosens Bioelectron 49:204–209
Xiao XX, Ulstrup J, Li H, Wang ME, Zhang JD, Si PC (2014) Nanoporous gold assembly of glucose oxidase for electrochemical biosensing. Electrochim Acta 130:559–567
Zheng D, Vashist SK, Al-Rubeaan K, Luong JT, Sheu FS (2012) Rapid and simple preparation of a reagentless glucose electrochemical biosensor. Analyst 137:3800–3805
Raitman OA, Katz E, Buckmann AF, Willner I (2002) Integration of polyaniline/poly(acrylic acid) films and redox enzymes on electrode supports: An in situ electrochemical/surface plasmon resonance study of the bioelectrocatalyzed oxidation of glucose or lactate in the integrated bioelectrocatalytic systems. J Am Chem Soc 124:6487–6496
Lad U, Kale GM, Bryaskova R (2013) Glucose oxidase encapsulated polyvinyl alcohol-silica hybrid films for an electrochemical glucose sensing electrode. Anal Chem 85:6349–6355
Hu CY, Yang DP, Zhu FJ, Jiang FJ, Shen SY, Zhang JL (2014) Enzyme-labeled Pt@BSA nanocomposite as a facile electrochemical biosensing interface for sensitive glucose determination. ACS Appl Mater Interfaces 6:4170–4178
Piao Y, Han DJ, Seo TS (2014) Highly conductive graphite nanoparticle based enzyme biosensor for electrochemical glucose detection. Sens Actuators B 194:454–459
Tao JZ, Xu GR, Hao HL, Yang FX, Ahn KS, Lee WY (2013) Poly(m-phenylenediamine)-Prussian blue hybrid film formed by one-step electrochemical deposition for glucose biosensor. J Electroanal Chem 689:96–102
Noiphung J, Songjaroen T, Dungchai W, Henry CS, Chailapakul O, Laiwattanapaisal W (2013) Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal Chim Acta 788:39–45
Chen W, Ding Y, Akhigbe J, Bruckner C, Li CM, Lei Y (2010) Enhanced electrochemical oxygen reduction-based glucose sensing using glucose oxidase on nanodendritic poly meso-tetrakis(2-thienyl)porphyrinato cobalt(II)-SWNTs composite electrodes. Biosens Bioelectron 26:504–510
Yu Y, Chen Z, He S, Zhang B, Li X, Yao M (2014) Direct electron transfer of glucose oxidase and biosensing for glucose based on PDDA-capped gold nanoparticle modified graphene/multi-walled carbon nanotubes electrode. Biosens Bioelectron 52:147–152
Chen C, Xie QJ, Yang DW, Xiao HL, Fu YC, Tan YM, Yao SZ (2013) Recent advances in electrochemical glucose biosensors: a review. RSC Adv 3:4473–4491
Chen XM, Wu GH, Cai ZX, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Si P, Huang YJ, Wang TH, Ma JM (2013) Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv 3:3487–3502
Huang HY, Chen PY (2010) PdNi- and Pd-coated electrodes prepared by electrodeposition from ionic liquid for nonenzymatic electrochemical determination of ethanol and glucose in alkaline media. Talanta 83:379–385
Wang GF, He XP, Wang LL, Gu AX, Huang Y, Fang B, Geng BY, Zhang XJ (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Lee J, Park SM (2005) Direct electrochemical assay of glucose using boron-doped diamond electrodes. Anal Chim Acta 545:27–32
Gougis M, Tabet-Aoul A, Ma D, Mohamedi M (2014) Laser synthesis and tailor-design of nanosized gold onto carbon nanotubes for non-enzymatic electrochemical glucose sensor. Sens Actuators B 193:363–369
Wu GH, Song XH, Wu YF, Chen XM, Luo F, Chen X (2013) Non-enzymatic electrochemical glucose sensor based on platinum nanoflowers supported on graphene oxide. Talanta 105:379–385
Meng L, Jin J, Yang GX, Lu TH, Zhang H, Cai CX (2009) Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal Chem 81:7271–7280
Quan H, Park SU, Park J (2010) Electrochemical oxidation of glucose on silver nanoparticle-modified composite electrodes. Electrochim Acta 55:2232–2237
Chen J, Zhang WD, Ye JS (2008) Nonenzymatic electrochemical glucose sensor based on MnO2/MWNTs nanocomposite. Electrochem Commun 10:1268–1271
Li CL, Wang HJ, Yamauchi Y (2013) Electrochemical deposition of mesoporous Pt-Au alloy films in aqueous surfactant solutions: towards a highly sensitive amperometric glucose sensor. Chem Eur J 19:2242–2246
Gao HC, Xiao F, Ching CB, Duan HW (2011) One-step electrochemical synthesis of PtNi nanoparticle-graphene nanocomposites for nonenzynnatic amperometric glucose detection. ACS Appl Mater Interfaces 3:3049–3057
Zhao J, Wei L, Peng C, Su Y, Yang Z, Zhang L, Wei H, Zhang Y (2013) A non-enzymatic glucose sensor based on the composite of cubic Cu nanoparticles and arc-synthesized multi-walled carbon nanotubes. Biosens Bioelectron 47:86–91
Nie HG, Yao Z, Zhou XM, Yang Z, Huang SM (2011) Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens Bioelectron 30:28–34
Sun F, Li L, Liu P, Lian YF (2011) Nonenzymatic electrochemical glucose sensor based on novel copper film. Electroanalysis 23:395–401
Liu Y, Teng H, Hou H, You T (2009) Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens Bioelectron 24:3329–3334
Mu Y, Jia DL, He YY, Miao YQ, Wu HL (2011) Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens Bioelectron 26:2948–2952
Lu LM, Zhang L, Qu FL, Lu HX, Zhang XB, Wu ZS, Huan SY, Wang QA, Shen GL, Yu RQ (2009) A nano-Ni based ultrasensitive nonenzymatic electrochemical sensor for glucose: enhancing sensitivity through a nanowire array strategy. Biosens Bioelectron 25:218–223
Yang J, Yu JH, Rudi Strickler J, Chang WJ, Gunasekaran S (2013) Nickel nanoparticle-chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens Bioelectron 47:530–538
Choi T, Kim SH, Lee CW, Kim H, Choi SK, Kim E, Park J (2015) Synthesis of carbon nanotube-nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens Bioelectron 63:325–330
Davis J, Moorcroft MJ, Wilkins SJ, Compton RG, Cardosi MF (2000) Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 125:737–741
Niu X, Lan M, Zhao H, Chen C (2013) Highly sensitive and selective nonenzymatic detection of glucose using three-dimensional porous nickel nanostructures. Anal Chem 85:3561–3569
Niu XH, Li YX, Tang J, Hu YL, Zhao HL, Lan MB (2014) Electrochemical sensing interfaces with tunable porosity for nonenzymatic glucose detection: A Cu foam case. Biosens Bioelectron 51:22–28
Zhang Y, Xiao XP, Sun YJ, Shi Y, Dai HC, Ni PJ, Hu JT, Li Z, Song YH, Wang L (2013) Electrochemical deposition of nickel nanoparticles on reduced graphene oxide film for nonenzymatic glucose sensing. Electroanalysis 25:959–966
Fan D, Wu S, Tian S, Zhou J, Ju Y, Ma C, Shi J (2014) Detection of dopamine on a poly(metanilic acid) decorated two-dimensional gold cavity array electrode. RSC Adv 4:49560–49568
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No.81302743), Guangxi Natural Science Foundation (No.2014GXNSFAA118031), and Youth Science Foundation of Guangxi Medical University (No. GXMUYSF201204).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 134 kb)
Rights and permissions
About this article
Cite this article
Zhong, A., Luo, X., Chen, L. et al. Enzyme-free sensing of glucose on a copper electrode modified with nickel nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182, 1197–1204 (2015). https://doi.org/10.1007/s00604-014-1443-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1443-y