Abstract
We report on the preparation of water-soluble and fluorescent silver/gold alloy nanoclusters (Ag/Au NCs) by a galvanic replacement reaction. The alloy NCs have an average diameter of 1.5 nm and are shown to be viable fluorescent probes for the determination of lead(II) due to aggregation-induced quenching of fluorescence. The alloy NCs are characterized in terms of photoluminescence spectrum, photoluminescence excitation spectrum, transmission electron microscopy and X-ray photoelectron spectroscopy which confirm the presence of alloy NCs. Bovine serum albumin, a well known stabilizing agent, has a high-affinity site for Pb2+ ion, and the resulting complex acts as a quencher of fluorescence. This finding has led to a method for selective determination of Pb2+ with a limit of detection of about 2 nM.

A practical and robust synthesis of water-soluble and fluorescence active, small-size Ag/Au alloy nanoclusters are successfully developed through galvanic replacement reaction. As-prepared alloy nanoclusters indicate excellent selectivity and sensitivity for Pb2+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy-metal ions, such as mercury(II) (Hg2+) and lead(II) (Pb2+), can result in serious and permanent damage to human organs due to their accumulative characters in the environment and biota [1, 2]. Therefore, detection of these heavy-metal ions is central to the metal monitoring of water or soil. In the past decade, some probes and/or schemes had been designed to detect heavy-metal ions selectively and sensitively due to the biotoxicity and bioaccumulative properties of heavy-metal ions which can cause damage to the kidney, liver, and nervous system and pose severe effects on human health [3–6]. Although some probes and methods (functional gold nanoparticles and quantum dots) for Pb2+ detection had been reported, highly sensitive and selective detection of Pb2+ in water is essential [7, 8]. On the other hand, DNAzyme exhibited high sensitivity for Pb2+ [9]. However, the expensive price made them unsuitable for many practical applications. Hence from the viewpoint of practical applications, an excellent probe should be not only highly sensitive and selective but also simple and economical in operation simultaneously.
Metal nanoclusters (NCs), an new class of fluorescent nanomaterials, was defined as isolated particles less than 2 nm in size with several to a hundred atoms [10]. Metal NCs with discrete energy levels showed molecule–like electronic transitions within the conduction band, and exhibited unique physical and chemical properties [11]. Similar to quantum dots, metal NCs exhibited size-dependent tunable fluorescence from visible to near-infrared regions with high quantum yields [12, 13]. Fluorescence NCs have been proved to be a more-powerful optical technique for the detection of low concentration analytes, owing to their handily preparation, low expense, multifunctional surface chemistry and rapid implementation [14–17]. For example, Yu groups reported luminescent Au NCs for the sensitive detection of Cu2+ [15]; Lin and coworkers used fluorescent Ag NCs as effective probes for highly selective detection of Hg2+ at ppb levels [16]; Pradeep and co-workers prepared blue fluorescent Cu NCs which can detect highly toxic Pb2+ ions in water [17]. Prior to our study, these fluorescent probes were usually based on metal NCs with single component and precise number of atoms. However, there are some challenges to prepare fluorescent alloy NCs as probe for detection of heavy-metal ions.
Alloy NCs have same advantages as metal NCs: photoluminescence, facile synthesis, good biocompatibility and multifunctional surface chemistry [18]. Alloy NCs had attracted much attention due to their unique physical and chemical properties, and practical applications in various areas [19]. While there are some technical issues in preparing metal alloy NCs, such as time-consuming preparation process and low quantum yields [20, 21]. Until now, several research groups have been trying a few routes to prepare alloy NCs. Among these approaches, galvanic replacement reaction is one of particularly good methods due to the high efficiency and simplicity [22]. This method had been applied in large-scale synthesis of various shaped alloy metals nanostructures [23]. Yet, few researches about fluorescent alloy NCs appeared through this way [24].
Herein, we applied the galvanic replacement reaction to achieve Ag/Au alloy NCs with red fluorescence. As-prepared Ag/Au alloy NCs were protected by bovine serum albumin (BSA) which could work as an important scaffold to prevent NCs from growing large NPs [25]. More importantly, NCs stabilized by BSA exhibited good dispersion and high stability in aqueous solution [26, 27]. These advantages illustrated that the resultant alloy NCs were promising fluorescent probes for detection of heavy-metal ions.
Experimental
Materials
Reduced glutathione (GSH, molecular weight of 307 g·mol−1), bovine serum albumin (BSA) were purchased from Aldrich (http://www.sigmaaldrich.com). Tetrachloroauric (III) acid (HAuCl4), silver nitrate (AgNO3, 99 %), hydrazine hydrate (N2H4 .2H2O, 85 wt.%), sulfuric acid (H2SO4) and isopropanol were analytical grade. All reagents were used as received without further purification. De-ionized water was used in all experiments.
The synthesis of Ag/Au alloy nanoclusters
The template of Ag nanoclusters (NCs) was prepared by chemical reducing method using N2H4 .2H2O as reducing agent, which was the similar as previous report [16]. The galvanic replacement reaction route was used to synthesize Ag/Au alloy NCs, which was similar to our previously reported synthetic procedure for Ag/Au alloy NCs with a slight improvement [22]. The 100 mg BSA, 0.25 mL Ag NCs (10 mmol·L−1) and 0.25 mL HAuCl4 (50 mmol·L−1) was dissolved in 4.5 mL aqueous solution under magnetic stirring. Then, 15 μL NaOH (1 mol·L−1) solution was introduced and the mixture was allowed to incubate at 40 °C under vigorous magnetic stirring for 4 h. The color of the solution changed from light yellow to deep brown. The purification of resultant BSA–Ag/Au alloy NCs was centrifuging at 12,000 rpm to remove AgCl precipitate. Then the resultant BSA–Ag/Au alloy NCs were precipitated by addition of H2SO4 (1 mol·L−1), collected through centrifugal at 8,000 r·min−1, and then re-dispersed in 10 mL water solution for further application.
Application as Pb (II) probe
PbCl2 was used for the study of Pb2+ detection. A 2.4 mmol · L−1 stock solution of PbCl2 was prepared, from which various Pb2+ concentrations were prepared by serial dilution. For the quenching studies, aqueous solutions of BSA–Ag/Au alloy NCs and Pb2+ with different concentrations were mixed, and equilibrated for 5 min before the spectral measurements. To check the selectivity cations of this probe, we carried out other metal ions including K+, Na+, Ca2+, Mg2+, Ba2+, Ni2+, Co2+, Cd2+, Fe2+ and Fe3+. The operation was exactly similar conditions that were used for the detection of Pb2+.
Characterization methods
UV–Vis absorption spectra were obtained by using a Lambda 800 UV–Vis spectrophotometer. Photoluminescence experiments were performed with a Shimadzu RF–5,301 PC spectrofluorimeter. X–ray photoelectron spectroscopy (XPS) using Mg Kα excitation (1,253.6 eV) was collected in a VG ES-CALAB MKII spectrometer. Binding energy calibration was based on C 1 s at 284.6 eV. The Fourier transform infrared spectroscopy (FT–IR) was measured at wavenumbers ranging from 500 cm−1 to 4,000 cm−1 using a Nicolet Avatar 360 FT–IR spectrophotometer. The morphology and mean diameter of resultant Ag/Au alloy NCs were characterized by Hitachi H–800 transmission electron microscope (TEM) operating at 200 kV.
Results and discussion
In the past few decades, lead(II) (Pb2+) levels are typically measured by using atomic absorption spectroscopy, inductively coupled plasma mass spectrometry (ICP-MS), and high performance liquid chromatography (HPLC). The drawbacks of them are time-consuming, expensive, and/or required sophisticated equipments. Compared with them, chemical probe is a sensitive, simple and fast method for quantifying Pb2+ and has attracted much attention [28–31]. Table 1 showed the comparison of different probes for determination of Pb2+. It is apparent the Ag/Au alloy nanoclusters (NCs) could work as fluorescent probes for detection of Pb2+ with highly selective and sensitive.
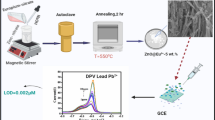
The synthesis of Ag/Au alloy NCs through galvanic replacement reaction route is schematically illustrated in Scheme 1. In the first step, Ag NCs, as the templates, were prepared by chemical-reduced method using N2H4 .2H2O as reducing agent and glutathione as stabling agent, which was similar to previous report [16]. Then, when the HAuCl4 and BSA were added to the aqueous solution of Ag NCs, the galvanic replacement reaction occurred due to standard reduction potential of AuCl4 −/Au pair (0.99 V vs SHE) was higher than that of Ag+/Ag pair (0.80 V vs SHE) [32]. In this process, the Ag (0) changes to Ag+ and form the AgCl in the solution. On the other hand, the AuCl4 − changes to Au (0). Herein the key for the formation of alloy NCs was controlled the molar ratios Ag/Au in galvanic replacement reaction. Theoretically speaking, reaction stoichiometry of Ag and AuCl4 − is at 3:1 [33]. Hence, when molar ratios Ag/Au is higher than this value, the product would be alloy NCs [22]. Besides, BSA play an important role in the stability of alloy NCs. BSA worked as the model protein to stabilize the clusters and provide steric protection due to the 17 disulfide bonds and 1 free cysteine, which had been widely used in the synthesis of fluorescent Ag and Au NCs [34, 35].
By adjusting molar ratios of Ag/Au at 5:1, Ag/Au alloy NCs would be formed. Resultant alloy NCs exhibited intense red fluorescence with peak maximum at 620 nm under a wavelength of excitation of 500 nm (Fig. 1a). Both the solution and the freeze-dried forms of clusters showed strong red emission under UV light (365 nm) (Fig. 1c and Fig. S1, Electronic Supplementary Material, ESM). The fluorescence quantum yields of resultant alloy NCs reached up to 2.4 % using Rodamine 6G (quantum yields, 0.95 in ethanol) as the standard, which was suitable for preparing fluorescent probes for detecting heavy-metal ions. Moreover, resultant alloy NCs had good dispersion in aqueous solution and there was no noticeable precipitation, which was attributed to capping layer of BSA to prevent NCs from agglomeration (Fig. 1b). Different from reported NCs, this newly developed Ag/Au alloy NCs can not give a well-defined excitation band (Fig. S2, ESM) [16].
The transmission electron microscope (TEM) image of the Ag/Au alloy NCs was shown in Fig. 2a. As indicated, this galvanic replacement method gave the average size of resulting alloy NCs was 1.5 ± 0.2 nm (Fig. 2b). Moreover, there was no formation of larger size nanoparticles or aggregation, which highlighted the unique template effect by BSA for the preparation of small-size alloy NCs. Moreover, the template of Ag NCs was too small to produce hollow or core-shell nanostructures, which was confirmed by high-resolution TEM image (inset of Fig. 2b). On the other hand, X–ray photoelectron spectroscopy (XPS) analysis is carried out to determine the oxidation state and elemental composition of as-prepared alloy NCs. In the present study, the binding energy of Ag 3d5/2 and Ag 3d3/2 appeared at 368.2 eV and 374.1 eV, which indicated the presence of Ag0 (Fig. 2c) [36]. The binding energy of Au 4f located at 88.1 eV and 84.3 eV respectively, could explicitly demonstrate that the electronic structure of Au in the alloy NCs was the coexistence of Au (0) and Au (I) (Fig. 2d) [37]. The results of photoluminescence, photoluminescence excitation, transmission electron microscopy and X-ray photoelectron spectroscopy confirmed one fact the Ag/Au alloy NCs were synthesized.
The surface chemistry of alloy NCs was confirmed by FT-IR spectroscopy. Comparing with the FT-IR spectra of alloy NCs and pure BSA, there were no differences in their spectra, inferring that the alloy NCs embedded in BSA would not affect the surface-structure of BSA (Fig. 3). BSA-capping fluorescent metal NCs displayed good dispersion and high stability in aqueous phase [26, 27]. Especially, BSA has a much-stronger affinity toward metal ions, including Pb2+, which is important for practical applications in ion detection. So with this fluorescence active, water soluble alloy NCs in hands, we then investigated their ability as probe in selective metal Pb2+ detection. It was found that the luminescence of alloy NCs was quenched in the presence of Pb2+ ion (Fig. 4a). The emission spectra displayed a gradual decrease in emission intensity with the increase in the solution Pb2+ concentration (Fig. 4). Moreover, as shown in Fig. 4c, a linear relationship was observed between the intensity of the fluorescence of resultant alloy NCs and the concentration of Pb2+ ions over the range from 0.5 nmol·L−1 to 100 nmol L−1, which based on the result of fluorescence spectra of Fig. 4a. The limit of detection (LOD) was estimated to be 2 nmol·L−1, which was much lower than the maximum safety level of Pb2+ (75 nmol L−1) in drinking water defined by the U.S. Environmental Protection Agency (EPA).
a, Changes in emission spectra (λex = 500 nm) of as-prepared Ag/Au alloy NCs upon the addition of Pb2+ ions in different concentrations. b Maximum emission intensity changes of a as a function of the solution Pb2+ concentration. c Expanded linear region of the calibration curve (0.5–100 nmol·L−1 of Pb2+)
To test the selectivity of this quenching behavior by Pb2+, other metal ions were used for the probe study, such as K+, Na+, Ca2+, Mg2+, Ba2+, Ni2+, Co2+ , Cd2+, Cu2+, Hg2+, Fe2+ and Fe3+. These ions were carried out as same as Pb2+ concentration (10−6 mol·L−1) and exactly similar study conditions. The PL intensity variation of alloy NCs toward a variety of metal ions was presented in Fig. 5 (Based on the fluorescence spectra of Fig. S3 and S4, ESM). The results clearly showed that little emission intensity changes were observed in the presence of these interference metal ions, which demonstrated that the assay method was highly selective for Pb2+ detection.
The reason for highly sensitive and selective fluorescence probe for Pb2+ is the aggregation-induced fluorescence quenching of Ag/Au alloy NCs. According to above discussion, the surface of alloy NCs was protected by BSA which was confirmed by FT-IR. This was important for highly selective and sensitive for Pb2+ detection, due to the alloy NCs aggregation induced by the complexation between BSA and the Pb2+ ion. BSA contains a high-affinity site for Pb2+ ion; the binding involves carboxylate groups [17]. The aggregation of alloy NCs induced by Pb2+ was confirmed by TEM image as shown in Fig. 6. We found that the Pb2+-induced aggregation of the as-prepared fluorescent alloy NCs completed and left only lager aggregation (Fig. 6a) with the size about 2.5 ± 0.5 nm (Fig. 6b), which caused the luminescence quenching of alloy NCs. This sensing mechanism based on aggregation-induced fluorescence quenching had been widely adopted for NCs as probe to determine metal ions [17, 38, 39].
It is well known that highly sensitive and selective detection of Pb2+ in water is crucial in environmental and biological fields. Herein, to evaluate whether the alloy NCs-based fluorescent probe is applicable to natural systems, drinking water solution and lake water collected from Taihu Lake at Wuxi City were investigated. The experimental results showed that although the fluorescence was affected in the real water samples due to the complex environment including the various cations and pH value, the fluorescence of as-prepared alloy NCs was quenched with increasing the Pb2+ concentration and the detection for Pb2+ was also highly sensitive (Fig. S5a and S5b, ESM) and a linear relationship was observed between the intensity of the fluorescence of resultant alloy NCs and the concentration of Pb2+ ions over the range from 5 to 20 nmol·L−1 (Fig. S5, ESM). These results further reveals that such a sensing system is highly selective toward Pb2+ over other compositions in real water samples.
Conclusions
A practical and robust synthesis of water-soluble and fluorescence active, small-size Ag/Au alloy nanoclusters (NCs) was successfully developed through galvanic replacement reaction. As-prepared alloy NCs exhibited red fluorescence with peak maximum at 620 nm and quantum yields up to 2.4 %. More importantly, the surface of as-prepared Ag/Au alloy NCs were protected by bovine serum albumin (BSA), which made them have good dispersion in aqueous solution and high stability. Besides, BSA have a much-stronger affinity toward metal ions, which suggested the resultant alloy NCs were promising fluorescent probes for detection of heavy-metal ions based on aggregation-induced fluorescence quenching mechanism. The results confirmed this newly developed alloy NCs indicated excellent selectivity and sensitivity for Pb2+ detection in water. Furthermore, the alloy NCs probe could detect Pb2+ ion in drinking water and lake water, which indicated that this novel sensing system has great potential for detection of Pb2+ in environmental samples.
References
Yuan ZQ, Peng MH, He Y, Yeung ES (2011) Functionalized fluorescent gold nanodots: synthesis and application for Pb2+ sensing. Chem Commun 47:11981–11983
Huang CC, Yang ZS, Lee KH, Chang HT (2007) Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew Chem Int Ed 46:6824–6828
Liu HY, Zhang X, Wu XM, Jiang LP, Burda C, Zhu JJ (2011) Rapid sonochemical synthesis of highly luminescent non-toxic AuNCs and Au@AgNCs and Cu (II) sensing. Chem Commun 47:4237–4239
Liu XJ, Zong CH, Lu LH (2012) Fluorescent silver nanoclusters for user-friendly detection of Cu2+ on a paper platform. Analyst 137:2406–2414
Morishita K, Maclean JL, Liu BW, Jiang H, Liu JW (2013) Correlation of photobleaching, oxidation and metal induced fluorescence quenching of DNA-templated silver nanoclusters. Nanoscale 5:2840–2849
Ho JA, Chang HC, Su WT (2012) DOPA-mediated reduction allows the facile synthesis of fluorescent gold nanoclusters for use as sensing probes for ferric Ions. Anal Chem 84:3246–3253
Xu H, Liu B, Chen Y (2012) A colorimetric method for the determination of lead(II) ions using gold nanoparticles and a guanine-rich oligonucleotide. Microchim Acta 177:89–94
Ali EM, Zheng YG, Yu HH, Ying JY (2007) Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal Chem 79:9452–9458
Zhang LB, Han BY, Li T, Wang EK (2011) Label-free DNAzyme-based fluorescing molecular switch for sensitive and selective detection of lead ions. Chem Commun 47:3099–101
Lu YZ, Chen W (2012) Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev 41:3594–3623
Shang L, Dong SJ, Nienhaus GU (2011) Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today 6:401–418
Richards CI, Choi S, Hsiang JC, Vosch YAT, Bongiorno A, Tzeng YL, Dickson RM (2008) Oligonucleotide-stabilized Ag nanocluster fluorophores. J Am Chem Soc 130:5038–5039
Zheng J, Zhou C, Yu MX, Liu JB (2012) Different sized luminescent gold nanoparticles. Nanoscale 4:4073–4083
Liu YL, Ai KL, Cheng XL, Huo LH, Lu LH (2010) Gold-nanocluster-based fluorescent sensors for highly sensitive and selective detection of cyanide in water. Adv Funct Mater 20:951–956
Chen Y, Wang Y, Wang CX, Li WY, Zhou HP, Jiao HP, Lin Q, Yu C (2013) Papain-directed synthesis of luminescent gold nanoclusters and the sensitive detection of Cu2+. J Colloid Interf Sci 396:63–68
Wang CX, Xu L, Wang Y, Zhang D, Shi XD, Dong FX, Yu K, Lin Q, Yang B (2012) Fluorescent silver nanoclusters as effective probes for highly selective detection of mercury(II) at parts-per-billion levels. Chem Asian J 7:1652–1656
Goswami N, Giri A, Bootharaju MS, Xavier PL, Pradeep T, Pal SK (2011) Copper quantum clusters in protein matrix: potential sensor of Pb2+ ion. Anal Chem 83:9676–9680
Udayabhaskararao T, Sun Y, Goswami N, Pal SK, Balasubramanian K, Pradeep T (2012) Ag7Au6: a 13-atom alloy quantum cluster. Angew Chem Int Ed 51:2155–2159
Yin F, Wang ZW, Palmer RE (2011) Controlled formation of mass-selected Cu-Au core-shell cluster beams. J Am Chem Soc 133:10325–10327
Andolina CM, Dewar AC, Smith AM, Marbella LE, Hartmannand MJ, Millstone JE (2013) Photoluminescent gold − copper nanoparticle alloys with composition-tunable near-infrared emission. J Am Chem Soc 135:5266–5269
Chowdhury S, Bhethanabotla VR, Sen R (2009) Effect of Ag-Cu alloy nanoparticle composition on luminescence enhancement/quenching. J Phys Chem C 113:13016–13022
Wang CX, Xu L, Xu XW, Cheng H, Sun HC, Lin Q, Zhang C (2014) Near infrared Ag/Au alloy nanoclusters: tunable photoluminescence and cellular imaging. J Colloid Interf Sci 416:274–279
Tong L, Cobley CM, Chen JG, Xia YN, Cheng JX (2010) Bright three-photon luminescence from gold/silver alloyed nanostructures for bioimaging with negligible photothermal toxicity. Angew Chem Int Ed 49:3485–3488
Mohanty JS, Xavier PL, Chaudhari K, Bootharaju MS, Goswami N, Pal SK, Pradeep T (2012) Luminescent, bimetallic AuAg alloy quantum clusters in protein templates. Nanoscale 4:4255–4262
Hu DH, Sheng ZH, Gong P, Zhang PF, Cai LT (2010) Highly selective fluorescent sensors for Hg2+ based on bovine serum albumin-capped gold nanoclusters. Analyst 135:1411–1416
Wang C, Wang CX, Xu L, Cheng H, Lin Q, Zhang C (2014) Protein-directed synthesis of pH-responsive red fluorescent copper nanoclusters and their applications in cellular imaging and catalysis. Nanoscale 6:1775–1781
Xie JP, Zheng YG, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888–889
Qi L, Shang Y, Wu F (2012) Colorimetric detection of lead(II) based on silver nanoparticles capped with iminodiacetic acid. Microchim Acta 178:221–227
Kim YJ, Johnson RC, Hupp JT (2001) Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal Ions. Nano Lett 1:165–167
Fei Y, Lv Z-Y, Wang A-J, Chen Y-H, Chen J-R, Feng J-J (2014) Simultaneous determination of trace levels of lead(II) and copper(II) by square wave stripping voltammetry using a glassy carbon electrode modified with hierarchical gold dendrites. Microchim Acta 181:389–394
Chen P, Greenberg B, Taghavi S, Romano C, Lelie D, He C (2005) An exceptionally selective lead(II)-regulatory protein from Ralstonia metallidurans: development of a fluorescent lead (II) probe angew. Chem Int Ed 44:2715–2719
Wang CX, Wang Y, Xu L, Shi XD, Li XW, Xu XW, Sun HC, Yang B, Lin Q (2013) A galvanic replacement route to prepare strongly fluorescent and highly stable gold nanodots for cellular imaging. Small 9:413–420
Sun YG, Xia YN (2004) Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J Am Chem Soc 126:3892–3901
Mathew A, Sajanlal PR, Pradeep T (2011) A fifteen atom silver cluster confined in bovine serum albumin. J Mater Chem 21:11205–11212
Yue Y, Liu TY, Li HW, Liu ZY, Wu YQ (2012) Microwave-assisted synthesis of BSA-protected small gold nanoclusters and their fluorescence-enhanced sensing of silver (Ι) ions. Nanoscale 4:2251–2254
Wang CX, Wang Y, Xu L, Zhang D, Liu MX, Li XW, Sun HC, Lin Q, Yang B (2012) Facile a queous-phase synthesis of biocompatible and fluorescent Ag2S nanoclusters for bioimaging: tunable photoluminescence from red to near infrared. Small 8:3137–3142
Chen Y, Zhou HP, Wang Y, Li WY, Chen J, Lin Q, Yu C (2013) Substrate hydrolysis triggered formation of fluorescent gold nanoclusters–a new platform for the sensing of enzyme activity. Chem Commun 49:9821–9823
Adhikari B, Banerjee A (2010) A facile synthesis of water-soluble fluorescent silver nanoclusters and HgII sensing. Chem Mater 22:4364–4371
Qu F, Li NB, Luo HQ (2012) Polyethyleneimine-templated Ag nanoclusters: a new fluorescent and colorimetric platform for sensitive and selective sensing halide ions and high disturbance-tolerant recognitions of iodide and bromide in coexistence with chloride under condition of high ionic strength. Anal Chem 84:10373–10379
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.50925207, No. 51,373,061), the Natural Science Foundation of Jiangsu Province, China (BK20140157), Programme of Introducing Talents of Discipline to Universities (111 Project B13025), and the Fundamental Research Funds for the Central Universities (JUSRP11418).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 464 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Cheng, H., Sun, Y. et al. Nanoclusters prepared from a silver/gold alloy as a fluorescent probe for selective and sensitive determination of lead(II). Microchim Acta 182, 695–701 (2015). https://doi.org/10.1007/s00604-014-1375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1375-6