Abstract
We describe a highly sensitive method for the determination of traces of xanthine based on the aggregation of citrate-stabilized gold nanoparticles (AuNPs). It is found that, under optimal conditions of pH, the imide group of xanthine is adsorbed on the surface of the AuNPs, thereby displacing citrate ions. This leads to an aggregation of the AuNPs via hydrogen-bond interactions. As a result, the color of the solution changes from red to blue which can be seen with bare eyes and also can be measured by spectrophotometry. The ratio of the absorbances at 630 nm and 520 nm is linearly related to the concentration of xanthine in the 125 nM to 6.0 μM range (r = 0.9988), and the detection limit (3σ/slope) is 23 nM. The method is simple, feasible and fast.

We describe a highly sensitive colorimetric method for the determination of traces of xanthine based on the aggregation of citrate-stabilized gold nanoparticles (AuNPs). The ratio of the absorbances at 630 nm and 520 nm is linearly related to the concentration of xanthine in the 125 nM to 6.0 μM range, and the detection limit is 23 nM. The method is simple, feasible and fast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xanthine (3, 7-dihydro-purine-2, 6-dione) is a metabolic product of adenine and guanine, presents in most human body tissues and fluids [1]. As the precursor of uric acid, abnormal level of xanthine in body has been found to be closely related to many diseases, including hyperuricemia, gout, xanthinuria, perinatal asphyxia and pre-eclampsia [2–6]. Meanwhile, xanthine is also an important biomarker in the food industries [7]. Nowadays, based on the formation of hydrogen peroxide (H2O2) according to the xanthine oxidase (XOD) catalyzed reaction, the detection of xanthine has been developed generally based on the electrochemical method [8–11]. Although these methods make great contributions to xanthine detection, most of them are suffer from cumbersome, time consuming, and complicated laboratory procedures. Therefore, it is significant to develop a simple and sensitive method for the xanthine sensing.
Noble metal nanomaterials, especially gold nanoparticles (AuNPs) have attracted much attention in science and biotechnology area due to their unique chemical and physical properties [12]. The well-dispersed AuNPs solution shows red color, whereas the color will changes from red to blue or purple once AuNPs aggregate [13]. Based on the distinct color change, AuNPs have been employed as a signal transducer on varieties of platforms for many analytes, such as DNA, protein, small molecule and metal ion [14–17]. The major advantage of AuNPs based methods is that the detection event can be simply transformed into color change, which can be easily observed by the bare eye without use other complicated or expensive instruments. More importantly, the extraordinarily high extinction coefficient of AuNPs enables the colorimetric methods have extremely high sensitivity [18].
Nowadays, some colorimetric methods for xanthine are emerging. For example, Wang et al. have reported a colorimetric method for xanthine by using BSA-stabilized Au clusters as peroxidase mimetics [19]. In their detection case, BSA-stabilized Au clusters catalyzed the peroxidase substrate 3, 3, 5, 5-tetramethylbenzidine (TMB) to form blue charge-transfer complex when xanthine was catalyzed by XOD to product H2O2. The method has good results, however, long analysis time, complicated procedures and special skills are required. In this work, we found the color of AuNPs change from red to blue after the addition of xanthine. Based on the experimental phenomenon, a new colorimetric method for xanthine was established. The method is simple, feasible and the detection can be completed within 15 min at the room temperature.
Experimental
Materials and reagents
Chloroauric acid tetrahydrate (HAuCl4 · 4H2O) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, www.sangon.com). Trisodium citrate dihydrate (Na3C6H5O7 · 2H2O), Vitamin c, uric acid and urea were ordered from Chengdu Kelong Chemical Reagent Co., Ltd. (Chengdu, China, www.cdkelong.com). Xanthine, XOD and hypoxanthine were purchased from Sigma (St. Louis, MO, USA, www.sigmaaldrich.com). Other reagents were of analytical reagent grade and used without further purification. Stock solution of xanthine (0.1 mM) was prepared by dissolving the amount of xanthine with distilled water and stored in a refrigerator at 4 °C. Working solutions were obtained via dilution the stock solution prior to use. Milli-Q purified water (18.2 MΩ · cm) was used throughout the experiments. The pH of the solutions was adjusted by Britton—Robinson (BR) buffer.
Apparatus
The absorption spectra of AuNPs were measured on a TU-1901 UV–vis spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China, http://www.pgeneral.com). Dynamic light scattering (DLS) and zeta (ζ)-potential analyses were completed on a DLS Zetasizer Nano-ZS90 System (Malvern, UK, www.malvern.com.cn). Vortex mixer XW-80A (Shanghai Jingke Industrial Co., Ltd. Shanghai, China, http://www.jk18.com) was employed to blend the solution. All measurements were taken at room temperature.
Synthesis of AuNPs
The synthesis of AuNPs were identical with our previous works by reducing HAuCl4 with trisodium citrate [16, 20]. All glassware used in the following procedures was thoroughly cleaned with freshly prepared aqua regia solution (1:3 HNO3/HCl), rinsed with water and dried before to use. Briefly, 4 mL 1 % (w/w) HAuCl4 solution was added into the 96 mL distilled water and heated to boiling with stirring. Then, 2 mL 5 % (w/w) Na3C6H5O7 · 2H2O was quickly added with vigorous stirring, which result in a color change from pale yellow to red within 3 min. The solution was kept boiling continued for 5 min, then naturally cooled to room temperature, and put in refrigerator (4 °C) for reserve. The resulting solution of AuNPs concentration was calculated to be 11.05 nM according to Beer’s law [21].
Procedure for xanthine detection
BR buffer, appropriate concentration of xanthine, and 2.21 nM AuNPs were subsequently added into the 1.5 mL centrifugal tube, vortex-mixed and incubated at room temperature for 5 min. The UV–vis absorption spectra were recorded over the wavelength range from 300 nm to 800 nm. The selectivity for xanthine was confirmed by adding other species instead of xanthine in the same way.
Results and discussion
Sensing principle for xanthine
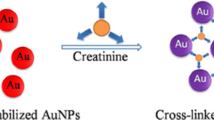
Articles have reported that imide group can bind to the surfaces of AuNPs easily than amino and hydroxyl groups [20, 22], thus we aim to develop a novel colorimetric method for xanthine (contained imide group). The detection mechanism is schematically depicted in Scheme 1. AuNPs used in this work were prepared using the standard trisodium citrate reduction, so the AuNPs surfaces capped plenty of citrate ions. The negatively charged citrate ions on the AuNPs surfaces provided sufficient electrostatic repulsion between the particles, and thus AuNPs were dispersed well and the color of the solution was red. In the presence of xanthine, however, imide group of xanthine (red part) readily bond to the AuNPs surfaces than the carboxyl groups of citrate ions and displaced citrate ions, and farther crosslinking of neighboring nanoparticles through hydrogen-bonding interactions. As a result, aggregation of AuNPs occurred, resulting in a red to blue color change. The color change could be seen with the bare eye and monitored by UV–vis spectrometer. Therefore, a simple and colorimetric method for xanthine detection can be carried out.
Feasibility of the method for xanthine detection
In order to identify the feasibility of the method for xanthine, a series of investigations have been made. Figure 1 shows the UV–vis absorption spectra of AuNPs at different conditions. Clearly, in the absence of xanthine, the AuNPs were stable and showed a red color (Fig. 1, Bottle a), which displayed a maximal absorption peak at 520 nm (Fig. 1, Curve a). However, the presence of xanthine led to a distinct color change from red to blue (Fig. 1, Bottle b), accompanying a new absorption peak at about 630 nm (Fig. 1, Curve b), which was ascribed to the aggregation of AuNPs.
In addition, ζ-potential and DLS analyses were used to confirm the state of AuNPs in the reaction solution. At pH 7.0, the ζ-potential and hydrodynamic diameter of the negatively charged AuNPs were determined to be −38.3 mV and 29.32 nm, respectively. After addition of 2 μM xanthine, the ζ-potential was changed to −37.2 mV and a larger aggregate size of 265.6 nm was obtained. The phenomenon can be ascribed to that xanthine bond to the AuNPs surfaces with the displacement of citrate ions, then aggregation of AuNPs occurred due to the screen of surface charges and cross-linked neighboring nanoparticles.
Effect of pH
The pKa1 of citric acid is 3.13, thus the AuNPs can be stabilized by the negatively charged carboxyl groups in citrate ions when pH is higher than 3.13 [23]. Figure 2 shows the influence of pH over the range from 6.8 to10.88. It could be found that the A 630/A 520 almost remains unchanged in the absence of xanthine (curve a in Fig. 2), which illustrates that the pH has little effect on the aggregation of AuNPs. Addition of 3.0 μM xanthine could obviously change the A 630/A 520. The A 630/A 520 decreased gently over the pH range of 6.8–7.54, while decreased sharply in the range of 7.54–9.73. When the pH was higher than 9.73, the A 630/A 520 nearly a constant, which was possibly due to the xanthine deprotonated at high pH (the pKa of xanthine is 7.7) [24], and could not effectively induce negatively charged AuNPs aggregation because of the strong electrostatic repulsion. At last, pH 7.0 was chosen as our experimental acidity.
Effect of incubation time
The aggregation kinetics of AuNPs with different concentrations of xanthine is exhibited in Fig. 3, which was performed by monitoring the A 630/A 520. As shown in Fig. 3, the A 630/A 520 gradually increased along with incubation time and quickly reached a maximum within several minutes. The results indicated that xanthine induce AuNPs aggregation almost complete within 5 min, so, 5 min was selected as the optimal time in this paper.
Sensitivity for xanthine detection
To quantitative detect xanthine under the optimal conditions, AuNPs without and with different concentrations of xanthine were tested. As shown in Fig. 4a, AuNPs dispersed well, which displayed a maximal absorption peak at 520 nm. However, in the presence of xanthine, two distinct peaks located at 520 nm and 630 nm were observed. With the increase of xanthine concentrations, the absorption peak located at 520 nm gradually decreased (Fig. 4a, Curves 1–15) and a peak located at 630 nm gradually increased (Fig. 4a, Curves 1–15). Visual color change (Fig. 4a, Insetted picture) was consistent with the absorption spectral measurements. Particularly, as low as 2.0 μM xanthine (red circle in picture) could be easily monitored by the bare eye without use other instruments. The intensity of A 630/A 520 was plotted as a function of xanthine concentrations. Figure 4b shows A 630/A 520 was increased gradually when xanthine concentration was raised from 125 nM to 6.0 μM. The linear equation could be expressed as A 630/A 520 = 0.07142 + 0.2211c xanthine (μM) with a correlation coefficient of 0.9988. The limit of the detection (defined as 3σ/slope) was estimated to be 23 nM (where σ was the relative standard deviation of a blank solution, n = 11).
(a) UV–vis absorption spectra of AuNPs in the presence of different concentrations of xanthine. The arrows indicate the signal changes with the increase of xanthine concentrations. (b) The plot of A 630/A 520 vs. the concentrations of xanthine from 125 nM to 6.0 μM. The error bars represent the standard deviation from three independent measurements. Concentrations: AuNPs, 2.21 nM; xanthine (from curves 1–15: 0, 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0 μM); pH 7.0
The analytical performance of the developed colorimetric method has been compared with other detection methods in Table 1. It can be seen that our method is simple and shows a lower detection limit.
Selectivity for xanthine detection
To examine the selectivity of the method towards xanthine, varieties of other compounds were investigated in this paper. It is well known that hypoxanthine can be catalyzed to xanthine and xanthine can further be catalyzed to uric acid based on xanthine oxidase (XOD) sequential oxidation. Because hypoxanthine do not contain imide group, so it cannot adsorb on the AuNPs surfaces and hence do not induce AuNPs aggregation. Meanwhile, although uric acid contains imide group, it is negatively charged at pH 7.0 due to the pK a of uric acid is about 5.5 [25] and hence do not induce AuNPs aggregation. Figure 5 shows the color change and A 630/A 520 in the presence of xanthine and other interferences. It is clear that only xanthine showed a drastic color change from blue to red and exhibited the largest A 630/A 520, which clearly demonstrated the AuNPs aggregated obviously. Whereas almost no change of color and A 630/A 520 was observed in the presence of a considerable excess of interferences. These results implied that this method showed a highly selective response to xanthine.
Values of A 630/A 520 and a photographic images (inset) of the AuNPs in the absence and presence of different species. The error bars represent the standard deviation from three independent measurements. Concentrations: AuNPs, 2.21 nM; xanthine, 6.0 μM; glucose, vitamin c, urea, 1 mM; hypoxanthine, 0.4 mM; uric acid, 0.8 mM; XOD, 0.5 UmL−1; Na+, 5 mM; K+, 2.5 mM; Cu2+, 0.5 mM; Ca2+, Mg2+, Zn2+, Fe3+, 0.3 mM; pH 7.0
Application to sample analysis
Finally, in order to evaluate the practical applicability and accuracy of the method for the detection of xanthine, recoveries were carried out by analyzing the xanthine together with other species. The determination results resulting from the average of six determinations were shown in Table 2. The recoveries of this method were calculated to be in the range between 97.0 and 100.6 % under the optimal conditions. On the other hand, healthy urine obtained from Southwest Hospital was used as real samples. The urine samples were stored frozen until deproteinization and used directly after filtration. The concentration of xanthine was found to be 1.32 ± 0.40 μM, which is very close to reported values in urine sample [19, 26].
Conclusion
In summary, we have described a highly selective and sensitive method for the detection of trace amount of xanthine in this paper. This method is based on the imide group of xanthine easily adsorbs onto the AuNPs surfaces and displacement of weakly bound citrate ions, then induce AuNPs aggregation through hydrogen-bonding interaction. As a consequence, the color of the solution changes notably from red to blue, as a correlation with the concentration of xanthine. Compared with other methods, this method offers several advantages. First, as low as 23 nM xanthine could be easily monitored by UV-spectrophotometer, and 2.0 μM xanthine is visualized by the bare eye without use any other sophisticated instruments. Second, the method is enzyme free, which is facile and low-cost. Third, cumbersome and labor-intensive AuNPs modification procedures are avoided, so the method is fast and the detection can be completed within 15 min.
References
Luong JHT, Male KB, Nguyen AL (1989) Application of polarography for monitoring the fish postmortem metabolite transformation. Enzyme Microb Technol 11:277–282
Campion EW, Glynn RJ, Delabry LO (1987) Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 82:421–426
Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX (2003) A literature review of the epidemiology and treatment of acute gout. Clin Ther 25:1593–1617
Gok F, Ichida K, Topaloglu R (2003) Mutational analysis of the xanthine dehydrogenase gene in a Turkish family with autosomal recessive. Nephrol Dial Transplant 18:2278–2283
Banupriya C, Ratnakar DP, Mondal N, Vishnu B, Koner BC (2008) Can urinary excretion rate of malon-dealdehyde, uric acid and protein predict the severity and impending death in perinatal asphyxia. Clin Biochem 41:968–973
McMaster-Fay RA (2008) Pre-eclampsia-a disease of oxidative stress resulting from the catabolism of DNA (primarily fetal). Biosci Hypotheses 1:35–43
Carsol MA, Volpe G, Mascini M (1997) Amperometric detection of uric acid and hypoxanthine with Xanthine oxidase immobilized and carbon based screen-printed electrode. Application for fish freshness determination. Talanta 44:2151–2159
Mu S, Shi Q (2013) Xanthine biosensor based on the direct oxidation of xanthine at an electrogenerated oligomer film. Biosens Bioelectron 47:429–435
Zhang L, Lei J, Zhang J, Ding L, Ju H (2012) Amperometric detection of hypoxanthine and xanthine by enzymatic amplification using a gold nanoparticles- carbon nanohorn hybrid as the carrier. Analyst 137:3126–3131
Devi R, Yadav S, Pundir CS (2012) Amperometric determination of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/ polyaniline composite film bound xanthine oxidase. Analyst 137:754–759
Pei J, Li XY (2000) Xanthine and hypoxanthine sensors based on xanthine oxidaseimmobilized on a CuPtCl6 chemically modified electrode and liquid chromatog-raphy electrochemical detection. Anal Chim Acta 414:205–213
Schultz DA (2003) Plasmon resonant particles for biological detection. Curr Opin Biotechnol 14:13–22
Li H, Rothberg LJ (2004) Label-Free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J Am Chem Soc 126:10958–10961
Spain E, Kojima R, Kaner RB, Wallace GG, O’Grady J, Lacey K, Barry T, Keyes TE, Forster RJ (2011) High sensitivity DNA detection using gold nanoparticle functionalised polyaniline nanofibres. Biosens Bioelectron 26:2613–2618
Woravith C, Apichat I (2012) Visual and colorimetric detection of mercury (II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchim Acta 176:57–64
Pu WD, Zhao HW, Huang CZ, Wu LP, Xu D (2013) Visual detection of arginine based on the unique guanidino group-induced aggregation of gold nanoparticles. Anal Chim Acta 18:78–83
Li W, Nie Z, He K, Xu X, Li Y, Huang Y, Yao S (2011) Simple, rapid and label-free colorimetric assay for Zn2+ based on unmodified gold nanoparticles and specific Zn2+ binding peptide. Chem Commun 47:4412–4414
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862
Wang XX, Wu Q, Shan Z, Huang QM (2011) BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens Bioelectron 15:3614–3619
Xu D, Zhao HW, Huang CZ, Wu LP, Pu WD, Zheng JJ, Zuo Y (2011) Sensitive and selective detection of mercury (II) based on the aggregation of gold nanoparticles stabilized by riboflavin. J Nanosic Nanotechnol 12:3006–3010
Jin R, Wu G, Li Z, Mirkin CA, Schatz GC (2003) What controls the melting properties of DNA-linked gold nanoparticle assemblies. J Am Chem Soc 125:1643–1654
Roy B, Saha A, Nandi AK (2011) Melamine sensing through riboflavin stabilized gold nanoparticles. Analyst 136:67–70
Ling J, Sang Y, Huang CZ (2008) Visual colorimetric detection of berberine hydrochloride with silver nanoparticles. J Pharmaceut Biomed 47:860–864
Kulikowska E, Kierdaszuk B, Shugar D (2004) Xanthine, xanthosine and its nucleotides: solution structures of neutral and ionic forms, and relevance to substrate properties in various enzyme systems and metabolic pathways. Acta Biochim Pol 51:493–531
Bera RK, Anoop A, Raj CR (2011) Enzyme-free colorimetric assay of serum uric acid. Chem Commun 47:11498–11500
Kthiwala M, Affum AO, Perry J, Brajter-Toth A (2008) Direct measurements of xanthine in 2000-fold diluted xanthinuric urine with a nanoporous carbon fiber sensor. Analyst 33:810–816
Acknowledgments
This work was supported by the Chongqing Natural Science Foundation (CSTC, 2007BB0049) and the National Natural Science Foundation Committee of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pu, W., Zhao, H., Wu, L. et al. A colorimetric method for the determination of xanthine based on the aggregation of gold nanoparticles. Microchim Acta 182, 395–400 (2015). https://doi.org/10.1007/s00604-014-1342-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1342-2