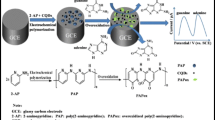
Abstract
A glassy carbon electrode (GCE) was modified with graphene quantum dots (GQDs) carrying silver nanoparticles. The modified GCE displays excellent performance in the electrochemical oxidation of guanine and adenine in showing lower anodic peak overpotentials (of 0.625 and 0.929 V, respectively) and increased anodic peak currents. The effective surface area of the modified GCE is about 21.5 times larger than that of the bare GCE. The electron transfer coefficient (α) and the electron transfer number (n) were calculated to be 2 and 0.60 for guanine, and 2 and 0.64 for adenine, respectively. Linear relationships between peak current and the concentrations were obtained in the range from 0.015 to 430 μM (for guanine) and 0.015 to 390 μM (for adenine). The detection limits are 10 nM and 12 nM (at a signal-to-noise ratio of 3), respectively. The modified GCE can well distinguish between guanine and adenine in mixed solutions, has fast response, a low detection limit, good reproducibility, and high stability.

A glassy carbon novel electrode modified with graphene quantum dots and silver nanoparticles was prepared. The modified electrode displays excellent performance in the electrochemical oxidation of guanine and adenine with fast response, a low detection limit, good reproducibility and stability
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials and nanotechnologies are providing numerous opportunities to promote gene determination and biomolecular sensing. Particularly, using nanomaterials as a medium of signal amplification attracts considerable attention. Graphene, due to its unique structural, electric, thermal, and mechanical properties, has shown several advantages in electrochemical biosensing [1–3]. Graphene quantum dots (GQDs), graphene sheets with lateral sizes of less than 100 nm, are a material with characteristics derived from both graphene and carbon dots [4–6]. Due to the fascinating optical, electronic properties, biocompatibility, low toxicity, and high water solubility, GQDs are demonstrated to be excellent probes for high contrast bioimaging and biosensing applications [7–10]. Recently, a comprehensive and detailed review about the application of graphene and graphene quantum dots in the fields of chemical and biochemical sensing had been reported by Gan et al. and Sun et al. [4, 11]. Metal nanoparticle has received considerable attention in recent years, such as Au, Ag, Pt, Pd nanoparticles. Ag nanoparticles not only have characteristics in common with noble metal nanoparticles, but also have unique properties such as the capacitance character, good electrical conductivity and high catalytic activity. Silver has the highest electrical conductivity among all metals and Ag nanostructures are considered to be instable. Moreover, Ag nanoparticles would be an ideal conductor to form electron-transferring channels between the target molecules and the electrode surface [12, 13].
Guanine and adenine are important bases found in DNA and RNA, which play a curial role in protein biosynthesis and the storage of genetic information [14]. The abnormal concentrations of these purines in cells may indicate the deficiency and mutation of the immunity system and the presence of various diseases, such as cancers and AIDS [15, 16]. In present, there is a series of methods for determination of guanine and adenine, such as high performance liquid chromatography, mass spectrometry, capillary electrophoresis and spectroscopic methods [17]. Although these methods display high sensitivity, there are some limitations in these methods, such as time-consuming, complex operation and expensiveness. In recent years, electrochemical methods provide useful alternatives due to their high sensitivity, fast response, inherent simplicity and low cost. For example, Liu et al. investigated the electrochemical behavior of guanine and adenine on a PbO2-carbon nanotubes-room temperature ionic liquid composite film modified glassy carbon electrode, which was useful for simultaneous and individual determination of guanine and adenine [18]. Liu et al. fabricated the polythionine/gold nanoparticles/multi-wall carbon nanotubes modified electrode and the modified electrode exhibited enhanced electrocatalytic behavior and good stability for the detection of guanine and adenine [19]. Huang et al. constructed a novel electrochemical sensor based on carboxylic acid functionalized graphene for simultaneous determination of adenine and guanine from DNA [20]. Hence, developing a simple, sensitive and reliable method for the simultaneous detection of adenine and guanine is essential to bioscience and clinical diagnosis.
In this paper, GQDs have been synthesized from carbon fibers by a simple method. Meanwhile, we developed a novel, simple and reliable electrochemical sensor based on the AgNPs and GQDs modified electrode for the determination of guanine and adenine. The sensor demonstrated favorable electrocatalytic activities in the oxidation towards guanine and adenine, and showed a fast response, high sensitivity and online detection ability. The sensor was successfully applied for the determination of guanine and adenine in denatured DNA and satisfactory results were obtained.
Materials and methods
Reagents
Guanine, adenine and fishing sperm DNA were purchased from Sigma- Aldrich (www.sigmaaldrich.com). Carbon fibers were purchased from Qinhuangdao Zichuan Carbon Fiber Co., Ltd (http://qhdzctxw168.1688.com). Silver nitrate was obtained from Tianjin Bes Chemical Industry Co., Ltd (www.tjbschem.com). Phosphate buffer solution was prepared by mixing the stock solution of 0.1 M NaH2PO4 and 0.1 MNa2HPO4 and adjusting the pH with 0.1 M H3PO4 or 0.1 M NaOH (www.aladdin-reagent.com). All other chemicals were of analytical grade and used as received without any further purification. Double distilled water was used throughout the studies.
Apparatus
Electrochemical experiments and the electrochemical impedance spectroscopy measurements were performed on a CHI-660E electrochemical workstation (Shanghai Chenhua Instrument Corporation, China. www.chinstr. com). A three-electrode cell was employed and a glassy carbon electrode (GCE) or a modified GCE was used as a working electrode. Platinum sheet was employed as counter electrode and the reference electrode was a saturated calomel electrode (www.gaossunion.cn). All potentials are referred to the saturated calomel electrode. Scanning electron microscopy image was determined with a JSM-6701 F field emission scanning electron microscope (Japanese Electron Optics Company. www.licp.cas.cn). High resolution- transmission electronic microscope image was obtained with a Tecnai G2 F30 S-Twin transmission electron microscope opened at an acceleration voltage of 300 kV (Philips-FEI company, Netherlands. www.licp.cas.cn).
Synthesis of graphene quantum dots
Graphene quantum dots were synthesized from carbon fibers by a simple method. Typically, carbon fibers (1.0 g) were dispersed in a mixture of concentrated H2SO4 and HNO3 (v:v = 3:1). The solution was sonicated for one hour and stirred for 20 h at 80 °C, 100 and 120 °C, respectively. After the completion of the reaction, double distilled water (about 500 mL) was then added to the dispersion. Finally, the pH was adjusted to 8 with Na2CO3. The final product was dialyzed in a dialysis bag for 5 days.
Fabrication of silver nanoparticles, graphene quantum dots and silver nanoparticles/graphene quantum dots modified electrodes
Before the modification, GCE were carefully polished to a mirror with 0.05 mm alumina slurry, rinsed thoroughly with double distilled water and then sonicated in HNO3 (1:1), ethanol (1:1), double distilled water. For the preparation of AgNPs/GCE, GQDs/GCE and AgNPs/GQDs/GCE, 10 mg GQDs was dispersed in 10 mL double distilled water and sonicated for 30 min to form a homogenous dispersion. Then, 5 μL of the dispersion was dropped on the pretreated GCE with a microsyringe, and dried for 12 h in air. The obtained GQDs/GCE was rinsed with distilled water to remove the loose adsorbed materials. Then the GQDs/GCE was immersed in 0.1 M potassium nitrate solution containing 3.0 × 10−3 M silver nitrate and electrochemical deposition of AgNPs was performed at 0.2 V for 90 s. The modified electrode was denoted as AgNPs/GQDs/GCE. Alternatively, different kinds of modified electrodes such as GQDs/GCE and AgNPs/GCE were also fabricated with a similar procedure.
Preparation of DNA samples
Thermally denatured DNA was obtained according to the reference report [21]. Briefly, the DNA sample was dissolved in double distilled water and then heated at 100 °C for 20 min, and finally the sample was quickly cooled in an ice bath.
Results and discussion
Characterization of graphene quantum dots
Figure 1 shows the typical high resolution-transmission electronic microscope image of GQDs. The spherical nanoparticles were observed and the diameters of these nanoparticles ranged between 5 nm and 20 nm. Meanwhile we can also observe the linear arrangement and better dispersion of the GQDs. When the GQDs were modified on the surface of electrode, the effective area of the modified electrode would be increased greatly. Moreover, because of the high electrical conductivity of the GQDs, the conductivity of the modified electrode enhanced significantly and this accelerated the electron transfer between the electrodes and target molecules.
Characteristics of the modified electrodes
The surface morphology of the silver nanoparticles/graphene quantum dots modified electrode
The surface morphology of the AgNPs/GQDs modified electrode was also observed by scanning electron microscopy and the image is shown in Fig. 2. There were plenty of globular shaped nanoparticles uniformly dispersed on the surface of GCE, which indicated that AgNPs and GQDs had been successfully modified on the surface of electrode and formed a dense conductive thin film. A slight agglomeration was observed, which could be attributed to the large surface area and small size effect of AgNPs and GQDs. The large surface area and small size effect resulted in increasing effective area of the electrode and well maintained electrocatalytic and conductive characteristics.
Electrochemical properties of the silver nanoparticles/graphene quantum dots modified electrode
Electrochemical impedance spectroscopy is an effective technique to probe the change of interface properties of an electrode surface during the modification process. Figure 3 shows the typical Nyquist plots for the bare GCE and modified electrodes in the presence of 5 mM [Fe(CN)6]3-/4- in 0.1 M KCl. The semicircular portion at higher frequencies represents the electron transfer limited process while a linear part at lower frequencies represents the diffusion limited process. At the bare GCE (curve a), it can be seen that the bare GCE showed a higher electron transfer resistance and the R et was obtained as 272.7 Ω by fitting the data using an appropriate equivalent circuit, which indicated that the electron transfer rate between the redox probe of [Fe(CN)6]3-/4- and electrode interface was very slow and blocked the electrochemical reaction of target molecules. At the AgNPs/GCE (curve b), the AgNPs/GCE exhibited a lower interfacial electron transfer resistance (204.6 Ω) than that of the bare electrode. The result showed that the presence of AgNPs facilitated the electron transfer between redox probe and electrode interface. However, at the GQDs/GCE (curve c), the GQDs/GCE showed a very low interfacial electron transfer resistance and the values of Rct decreased greatly to 63.0 Ω, which demonstrated that the GQDs/GCE could effectively improve the conductivity of the electrode and have higher electrochemical activity than bare GCE and AgNPs/GCE. The electron transfer ability was significantly improved due to the high conductivity of GQDs. While at the AgNPs/GQDs/GCE (curve d), a nearly straight line was obtained and the Rct value was decreased dramatically to 8.5 Ω, which indicated that the presence of AgNPs and GQDs could enhance significantly the conductivity and facilitated the electron transfer between probe and electrode interface.
Nyquist plots of (a) bare GCE, (b) AgNPs/GCE, (c) GQDs/GCE, (d) AgNPs/GQDs/GCE in the solution of 5.0 mM [Fe (CN)6]3-/4- with the frequencies swept from 105 to 0.1 Hz. Inset is the Randles circuit model for the modified electrodes. Rs: solution resistance; Rct: charge-transfer resistance; Zw: Warbury resistance; c: double-layer capacitance
Electrochemical behavior of adenine and guanine
The cyclic voltammograms of 110 μM guanine and adenine in 0.1 M phosphate buffer solution on four different electrodes are illustrated in Fig. 4. At the bare GCE (curve a), the guanine and adenine gave two small oxidation peaks at 0.684 V and 0.995 V, respectively and the cyclic voltammogram showed very low responses. No cathodic peaks were observed on the reverse scan within the investigated potential range, indicating totally irreversible electron-transfer kinetics for both electrode interfaces. Compared to the electrochemical response on the bare GCE, the peak current signals of guanine and adenine on the AgNPs/GCE (curve d) were enhanced significantly. Electrodeposited AgNPs would have more active surface silver atoms, which results in a higher current signal than that of the bulk silver. At the GQDs/GCE (curve c), the response signals further improved with their oxidative potential moving negatively, indicating that GQDs/GCE had a good electrocatalytic ability to the electrochemical reaction of guanine and adenine. The results obtained from experiments showed that the cyclic voltammetry was affected significantly by the modification with GQDs and that the contribution of the GQDs to the electrochemical signal and oxidative potential was quite large. However, at the AgNPs/GQDs/GCE (curve b), two well-defined oxidation peak were observed at 0.625 V and 0.929 V and that can be attributed to the two electron oxidative reaction of guanine and adenine. Meanwhile, there was an increase in the anodic current response and a decrease in the oxidative potential. Perhaps it is sufficient to say that a 3 fold improvement of the peak current was obtained and the oxidative potential shifted negatively 59 mV and 76 mV than that on the bare GCE, which indicated that the AgNPs and GQDs modified electrode showed good electrocatalytic behavior to guanine and adenine and the direct electron transfer of guanine and adenine on bare GCE was very fast. The peak-to-peak separation of guanine and adenine was 304 mV, which was large enough to determine guanine and adenine simultaneously without any interference. This catalytic effect was attributed to the presence and synergistic effect of the AgNPs and GQDs in the fabricated bioelectrode. Compared to the bare GCE, AgNPs/GCE and GQDs/GCE, the AgNPs and GQDs modified electrode possessed larger surface areas, high conductivity, uniform structure, good biocompatibility and anti-fouling ability.
Optimization of conditions
Deposition potential and deposition time
To obtain an excellent microenvironment for the electrochemical detection of guanine and adenine, silver nanoparticles deposition time and deposition potential were studied. As shown in Fig. S1 (Electronic Supplementary Material, ESM), the oxidation peak currents of guanine and adenine increased gradually and then decreased with the deposition time increased from 0 to 120 s and the maximum response current was obtained at 90 s. When the deposition potential shifting positively from −0.1 to 0.3 V, the response current reached the maximum value at 0.2 V, and then decreased as the deposition potential further increased to 0.3 V. The results indicated that the AgNPs/GQDs modified electrode has an excellent electrocatalytic performance and large effect area at −0.4 V for 90 s. Therefore, 90 s and −0.4 V was selected as the optimized value for the futher experiments.
Effect of the solution pH
A proton is always involved in the electrochemical reactions of organic compounds and exerts significant impact on the reaction speed. Therefore, the influences of solution pH values on the electrochemical behavior of the guanine and adenine were also investigated. Plots of peak current against pH are shown in Fig. S2A (ESM). When the pH increased from 4.0 to 7.0, the peak current gradually increased and then decreased. It is apparent that the highest peak currents were obtained at pH 4.5. However, this peak potential is also pH-dependent and shifts to lower potentials as pH is increased (Fig. S2B, ESM). It was found that the peak potential Epa was linear with pH value in the range of 4.0-7.0 and the linear regression equation was Epa (V) = 0.9108 - 0.046 pH for guanine and Epa (V) = 1.2514 - 0.049 pH for adenine, respectively. A shift of −46 mV for guanine and −49 mV for adenine with an increase of 1 pH unit is indicative of electron transfer coupled with proton transfer.
Effect of accumulation potential and time
To obtain a large response signal and improve the detection sensitivity, the influence of accumulation time and accumulation potential on the electrochemical behavior of guanine and adenine were also studied. Fig. S3A (ESM) shows that the peak current of guanine and adenine rapidly increased with increasing accumulation time from 0 to 100 s, and slowly increased with the further increase to 150 s. However, as the accumulation time further increased to 200 s, the peak current of guanine and adenine gradually decreased. The peak currents of guanine and adenine were about 2.2 and 2.0 times of that without accumulation, respectively. Therefore, an accumulation time of 150 s was chosen as optimum for the guanine and adenine to obtain stable peak with higher sensitivity and shorter analysis time. Fig. S3B (ESM) shows the effect of accumulation potential on the peak current of guanine and adenine after 150 s accumulation. The peak currents lightly increased and then decreased as the potentials shifted positively from −0.3 to 0.1 V. The maximal peak current was obtained at −0.2 V. Therefore, −0.2 V was applied as the optimal accumulation potential.
Effect of potential scan rate
The cyclic voltammograms for the AgNPs/GQDs/GCE at different scan rates were examined, as shown in Fig. 5. It was found that the oxidation peak current of guanine and adenine gradually increased with increasing scan rate and they are both proportional to the square root of scan rate in the range of 0.01 - 0.11 V s−1. Inset of Fig. 5 presents the relationship plot between the oxidation peak current and scan rate. The regression equations can be expressed as I pa (μA) = −0.4760 υ1/2 (mV1/2 s-1/2) - 3.7226 for guanine and I pa (μA) = −1.1798 υ1/2 (mV1/2 s-1/2) - 2.8310 for adenine with a correlation coefficient of 0.9915 and 0.9982, respectively, indicating the electrochemical reaction of guanine and adenine are a diffusion-controlled process, instead of a surface-reaction-controlled process.
The fact that peak potential is significantly dependent on scan rate suggests that reaction is electrochemically irreversible at the AgNPs/GQDs/GCE. There is a good linear relationship between the peak potential and the natural logarithm of scan rate. The anodic peak potential of guanine and adenine shifted to more positive potentials as the scan rate was increased and a good linear relationship between the peak currents and the natural logarithm of scan rate was obtained. The regression equations were Epa (V) = 0.0213 ln υ + 0.6498 (R 2 = 0.9936) for guanine and Epa (V) = 0.0202 ln υ + 0.9792 (R 2 = 0.9978) for adenine, respectively. According to Laviron’s theory [22], the relationship between Epa and scan rate could be expressed by the following equation:
where, n is the electron transfer number, α is the electron transfer coefficient, k 0 is standard rate constant of the reaction, E 0 is formal potential. R, F and T have their usual meanings (R = 8.314 J mol−1 K−1, F = 96480 C mol−1, T = 298 K). Thus, the value of αn can be easily calculated from the slope of Epa - ln υ. According to equation (1), the value of αn was calculated to be 1.21 for guanine and 1.27 for adenine. Generally, α is assumed to be 0.5 in totally irreversible electrode process. Therefore, the transfer electron number was found to be 2.42 for guanine and 2.54 for adenine. Combining with the result of Section 3.4.2, we know that the electrochemical oxidation of guanine and adenine on AgNPs/GQDs/GCE was a two-electron and two-proton process. The charge transfer coefficient was calculated to be 0.60 for guanine and 0.64 for adenine, respectively. The results indicated that GQDs and AgNPs was excellent promoter for the direct electron transfer between biomolecules and GCE. The probable reactions of guanine and adenine on AgNPs/GQDs/GCE were described as Scheme S1 (ESM).
Effective areas of the electrode
The effective areas of GCE and AgNPs/GQDs modified electrode were studied by chronocoulumetry using 0.1 mM K3[Fe(CN)6] as model complex. (The diffusion coefficient D of K3[Fe(CN)6] is 7.6 × 10−6 cm2 s−1 [23]). Fig. S4 (ESM) shows the plot of Q-t curve of the bare GCE and AgNPs/GQDs modified electrode. According to Eq. (2) given by Anson [24]
where n is the electron transfer number, F is the Faraday’s constant, A is the effective area of electrode, c is the concentration, D is the diffusion coefficient, Qdl is the double layer charge which could be eliminated by background subtraction, Qads is the Faradic charge. The value of the effective areas (A) of GCE and AgNPs/GQDs/GCE were found to be 0.103 cm2 and 2.207 cm2 from the slope of Q vs. t1/2. The effective surface areas of AgNPs/GQDs/GCE increased about 21.51 times larger than that on the bare GCE, which would increase the adsorption of guanine and adenine, leading to enhanced current response signal.
Analytical performance
The differential pulse voltammetry (DPV) is an effective and a rapid electroanalytical technique with lower concentration detection limits. In the present paper, the DPV was employed for the determination of guanine and adenine. Fig. 6 displays the DPV response of the AgNPs/GQDs modified electrode for increasing guanine and adenine concentrations under the optimum experimental conditions. Two well-defined oxidation peaks were observed. Meanwhile, a good linear relationship between the peak currents and the guanine/adenine concentration was obtained. At lower concentrations, the peak currents were proportional to guanine and adenine concentration in the range of 0.015-25 μM and 0.015-30 μM, respectively. The regression equation for guanine was I pa (μA) = −0.4046 C (μM) - 0.6787, with a correlation coefficient of 0.9911 and for adenine was I pa (μA) = −0.2679 C (μM) - 0.2086, with a correlation coefficient of 0.9990. At higher concentrations, the peak currents were proportional to guanine and adenine concentration in the range of 25–430 μM and 30–390 μM, respectively. The regression equations for guanine and adenine were I pa (μA) = −0.0387 C (μM) - 9.6740 (R 2 = 0.9983) and I pa (μA) = −0.0256 C (μM) - 14.1762 (R 2 = 0.9894). The detection limits were estimated to be 0.010 μM for guanine and 0.012 μM for adenine. The low detection limit of the AgNPs/GQDs modified electrode could be ascribed to the high loading of AgNPs and GQDs by the present method and the rapid electron transfer between guanine/adenine and the AgNPs/GQDs modified electrode. The comparison of AgNPs/GQDs with other modified electrodes for guanine and adenine detection is shown in Table 1. From the Table 1 it can be seen that this method had the comparable sensitivity, detection limit and wide linear range for the detection with the simple electrode preparation procedure. Thus, this method allowed both simultaneous and sensitive determination of guanine and adenine.
Reproducibility and stability
The reproducibility and stability of the AgNPs/GQDs modified electrode was also studied. The relative standard deviation (RSD) of the sensor response to 100 μM guanine is 2.3 % for ten successive assays. The results indicated that the prepared biosensor showed an acceptable repeatability. The fabrication reproducibility was examined at five different AgNPs/GQDs modified electrodes prepared under the same conditions, and the RSD of the sensor response to 100 μM guanine is 5.6 %. The long-term stability of AgNPs/GQDs modified electrodes was also evaluated, and 96.6 and 91.4 % of its initial current response to the oxidation of 100 μM guanine can be obtained after seven days and 3 weeks, respectively. These results suggest that the modified electrode has good long-term stability and reproducibility. The superior stability and reproducibility may be the result of the biocompatible microenvironment for the guanine provided the AgNPs/GQDs modified electrode.
Interferences
The practical usefulness of the sensor often depends upon the selectivity. Therefore, the determination of guanine and adenine (50 μM) in the presence of coexisting ions and other compounds in the biological sample was also investigated. The results indicated that 100-fold concentrations of K+, Na+, Ca2+, Mg2+, Fe2+, Al3+, Cl−, CO3 2−, SO4 2−, NO3 −, PO4 3−, glutamic acid, Uracil, glucose, Thymine, cysteine, do not affect guanine and adenine current response (signal change <5 %). 20-fold concentrations of L-tryptophan, L-cysteine, citric acid, glucose, L-leucine, dopamine barely influenced the current response of guanine and adenine. These results indicated that AgNPs/GQDs/GCE has an excellent anti-interference ability for some foreign substances and good selectivity for guanine and adenine detection, which might be applied to determine guanine and adenine in biological samples.
Analytical applications
In order to evaluate the validity of the method, the modified electrode was applied to determine guanine and adenine in thermally denatured DNA samples. Typically, the thermally denatured DNA (50 μL) was transferred to an electrolytic cell for the determination using DPV and two well-defined oxidation peaks were observed due to the oxidation of guanine and adenine in denatured DNA. Then, 100 μM of guanine and adenine standard solution was added and the peak currents of the guanine and adenine were recorded again. According to the differences between the peak currents of guanine and adenine, the concentration of guanine and adenine in thermally denatured DNA can be obtained by the calibration graph. The results were found to be 22.06 % and 27.94 % (molar ratio, mol %), respectively. Because of G = C, A = T in DNA, the value (G + C)/(A + T) of 0.79 was obtained for thermally denatured DNA, which coincide to the standard value of 0.77 [28]. The results indicated that the present method for practical samples was accurate and reliable.
Conclusions
A novel electrochemical approach for the simultaneous detection of guanine and adenine has been developed. The prepared AgNPs/GQDs modified electrode displayed excellent electrocatalytic properties in terms of fast current response, low detection limit, good reproducibility and high stability toward the electrochemical oxidation of guanine and adenine. The large peak separations obtained with this electrode allow it to simultaneously detect guanine and adenine with no mutual interference. Moreover, a remarkable improvement in the kinetics of the electron transfer for guanine and adenine was observed on the surface of the AgNPs/GQDs modified electrode, by shifting negatively in anodic peak potentials and significantly increasing the anodic peak current. The outstanding electrochemical and electrocatalytic performance, together with its inherent biocompatibility, make the AgNPs and GQDs composite film an interesting candidate for the study of the direct electrochemistry behaviors of redox substance. In addition, the AgNPs and GQDs composite film can be exploited in the construction of new electrochemical sensors.
References
Lu CH, Yang HH, Zhu CL, Chen X, Chen GN (2009) A graphene platform for sensing biomolecules. Angew Chem Int Ed 48:4785–4787
Alwarappan S, Erdem A, Liu C, Li CZ (2009) Probing the electrochemical properties of graphene nanosheets for biosensing applications. J Phys Chem C 113:8853–8857
Zhang Y, Wu CY, Zhou XJ, Wu XC, Yang YQ, Wu HX, Guo SW, Zhang JY (2013) Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 5:1816–1819
Sun HJ, Wu L, Wei WL, Qu XG (2013) Recent advances in graphene quantum dots for sensing. Mater Today 16:433–442
Shen J, Zhu Y, Yang X, Li C (2012) Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun 48:3686–3699
Li LL, Wu GH, Yang GH, Peng J, Zhao JW, Zhu JJ (2013) Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 5:4015–4039
Peng J, Gao W, Gupta BK, Liu Z, Romero-Aburto R, Ge LH, Song L, Alemany LB, Zhan XB, Gao GH, Vithayathil SA, Kaipparettu BA, Marti AA, Hayashi T, Zhu JJ, Ajayan PM (2012) Graphene quantum dots derived from carbon fibers. Nano Lett 12:844–849
Cheng HH, Zhao Y, Fan YQ, Xie XJ, Qu LT, Shi GQ (2012) Graphene quantum dot assembled nanotubes: A new platform for efficient Raman enhancement. ACS Nano 6:2237–2244
Tang LB, Ji RB, Cao XK, Lin JY, Jiang HX, Li XM, Teng KS, Luk CM, Zeng SJ, Hao JH, Lau SP (2012) Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 6:5102–5110
Zhao J, Chen GF, Zhu L, Li GX (2011) Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem Commun 13:31–33
Gan T, Hu SS (2011) Electrochemical sensors based on graphene materials. Microchim Acta 175:1–19
Mao SX, Li WF, Long YM, Tu YF, Deng AP (2012) Sensitive electrochemical sensor of tryptophan based on Ag@C core-shell nanocomposite modified glassy carbon electrode. Anal Chim Acta 738:35–40
Rounaghi G, kakhki RM, Azizi-toupkanloo H (2012) Voltammetric determination of 4-nitrophenol using a modified carbon paste electrode based on a new synthetic crown ether/silver nanoparticles. Mater Sci Eng C 32:172–177
Wang WP, Zhou L, Wang SM, Luo Z, Hu ZD (2008) Rapid and simple determination of adenine and guanine in DNA extract by micellar electrokinetic chromatography with indirect laser-induced fluorescence detection. Talanta 74:1050–1055
Zen JM, Chang MR, Ilangovan G (1999) Simultaneous determination of guanine and adenine contents in DNA, RNA and synthetic oligonucleotides using a chemically modified electrode. Analyst 124:679–684
Yang FQ, Guan J, Li SP (2007) Fast simultaneous determination of 14 nucleosides and nucleobases in cultured cordyceps using ultra- performance liquid chromatography. Talanta 73:269–273
Xiao F, Zhao FQ, Li JW, Liu LQ, Zeng BZ (2008) Characterization of hydrophobic ionic liquid-carbon nanotubes-gold nanoparticles composite film coated electrode and the simultaneous voltammetric determination of guanine and adenine. Electrochim Acta 53:7781–7788
Liu T, Zhu XB, Cui L, Ju P, Qu XJ, Ai SY (2011) Simultaneous determination of adenine and guanine utilizing PbO2-carbon nanotubes-ionic liquid composite film modified glassy carbon electrode. J Electroanal Chem 651:216–221
Liu HY, Wang GF, Chen DL, Zhang W, Li CJ, Fang B (2008) Fabrication of polythionine/NPAu/MWNTs modified electrode for simultaneous determination of adenine and guanine in DNA. Sensor Actuat B 128:414–421
Huang KJ, Niu DJ, Sun JY, Han CH, Wu ZW, Li YL, Xiong XQ (2011) Novel electrochemical sensor based on functionalized graphene for simultaneous determination of adenine and guanine in DNA. Colloid Surf B-Biointerfaces 82:543–549
Marmur J, Rownd R, Schildkraut CL (1963) Progress in nucleic acid research. Academic, New York
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Adams RN (1969) Electrochemistry at solid electrodes. Marcel Dekker, New York
Anson FC (1964) Application of potentiostatic current integration to the study of the adsorption of cobalt(III)-(ethylenedinitrilo(tetraacetate) on mercury electrodes. Anal Chem 36:932–934
Sun W, Li YZ, Duan YY, Jiao K (2008) Direct electrocatalytic oxidation of adenine and guanine on carbon ionic liquid electrode and the simultaneous determination. Biosens Bioelectron 24:988–993
Zou LN, Li YM, Ye BX (2011) Voltammetric sensing of guanine and adenine using a glassy carbon electrode modified with a tetraoxocalix[2] arene[2]triazine Langmuir-Blodgett film. Microchim Acta 173:285–291
Wei YL, Luo LQ, Ding YP, Liu X, Chu YL (2013) A glassy carbon electrode modified with poly(eriochrome black T) for sensitive determination of adenine and guanine. Microchim Acta 180:887–893
Davision N (1972) The Biochemistry of the Nucleic Acids, 7th edn. Cox & Nyman, Norfolk
Acknowledgments
This work was supported by the HongLiu Natural Science Foundation of Lanzhou University of Technology and the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 237 kb)
Rights and permissions
About this article
Cite this article
Wang, G., Shi, G., Chen, X. et al. A glassy carbon electrode modified with graphene quantum dots and silver nanoparticles for simultaneous determination of guanine and adenine. Microchim Acta 182, 315–322 (2015). https://doi.org/10.1007/s00604-014-1335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1335-1