Abstract
Electrochemiluminescence (ECL) integrates the advantages of electrochemical detection and chemiluminescent techniques. The method has received particular attention because it is highly sensitive and selective, has a wide linear range but low reagent costs. The use of nanomaterials with their unique physical and chemical properties has led to new kinds of biosensors that exhibit high sensitivity and stability. Compared to other nanomaterials, DNA nanostructures are more biocompatible, more hydrophilic, and thus less prone to nonspecific adsorption onto the electrode surface. We describe here a label-free and ultrasensitive ECL biosensor for detecting a cancer-associated microRNA at a femtomolar level. We have designed two auxiliary probes that cause the formation of a long-range self-assembly in the form of a μm-long 1-dimensional DNA concatamer. These can be used as carriers for signal amplification. The intercalation of the ECL probe Ru(phen)3 2+ into the grooves of the concatamers leads to a substantial increase in ECL intensity. This amplified sensor shows high selectivity for discriminating complementary target and other mismatched RNAs. The biosensor enables the quantification of the expression of microRNA-21 in MCF-7 cells. It also displays very low limits of detection and provides an alternative approach for the detection of RNA or DNA detection in diagnostics and gene analysis.

The long-range self-assembly DNA concatamers were used as carriers for signal amplification by the intercalation of numerous ECL probe (Ru(phen)3 2+) into the grooves of the DNA concatamers. Such signal amplification strategy lead to a substantial increase in ECL intensity and sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrogenerated chemiluminescence, also defined as electrochemiluminescence, is probably best described as chemiluminescence produced directly or indirectly in situ as the result of conversion of electrochemical energy into radiative energy at the surface of an electrode via controlling the applied potential at the working electrode [1]. This technique has received considerable attention during the past several decades owing to its versatility, simplified optical setup, and good control [2]. It has been used as an important detection method in trace amounts of metallic ions [3–5], cells [6], nucleic acids [7, 8] and proteins [9, 10], which are of great significance to researchers and clinicians.
Nanomaterials, due to their excellent properties, such as good conductivity, large specific surface area and special catalytic properties, have attracted great interest as ECL probe carriers in ECL biosensors. They can carry multiple ECL labels and produce a significant signal, which can enhance the sensitivity of the ECL biosensors. These nanomaterials employed in ECL encompass a broad range of materials including polymeric microbeads [11, 12], gold nanoparticles [13, 14], carbon nanotubes [15–17] and silica nanoparticles, etc. [18, 19]. Nanomaterials have broadened the prospects for the application of ECL biosensing.
As a vital part of modern nanotechnology, DNA self-assembly has attracted widespread interest since Seeman proposed the possibility of the use of DNA as a structural material for self-assembly in 1982 [20]. A key aspect of self-assembly is the use of sticky-ended cohesion by hydrogen bonding to combine pieces of linear duplex DNA. The dimensions of DNA are inherently on the nanoscale. Hence, construction involving DNA is fundamentally an exercise in nanoscience and nanotechnology [21]. Nowadays, many self-assembled DNA nanostructures have been fabricated [22–24] and applied in biosensing [25–27].
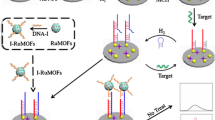
Motivated by the above observation, in this study, based on long-range self-assembled DNA nanostructures as carrier for ECL signal amplification, we fabricated a label-free and ultrasensitive electrochemiluminescence biosensor for detection of microRNA-21, which is a biomarker for various cancers and other diseases and has high expression in breast cancer [28], diffuse large B-cell lymphoma [29], ovarian cancer [30] and myocardial diseases [31]. The principle is based on the two designed auxiliary probes, auxiliary probe 1 (AP1) and auxiliary probe 2 (AP2), which can lead to long-range self-assembly and form micrometer-long DNA nanostructures (DNA concatamers) on the electrode surface in the presence of the target sequence. Ru(phen)3 2+ (phen = phenanthroline), which acts as ECL probe, can intercalate into the double stranded DNA (ds-DNA). It has been reported that the ds-DNA can be intercalated with Ru(phen)3 2+, and every four base pairs can embed one Ru(phen)3 2+ molecule [9]. After the successful intercalation of plentiful Ru(phen)3 2+, it generates significantly amplified ECL signals for the detection when using TPA as coreactant. As displayed in Fig. 1, the capture probe (CP) is modified with thiol and immobilized on the gold electrode (GE) by the interaction of Au-S. In the absence of the target microRNA, the capture probe maintained the stem-loop structure. Although the added auxiliary probe 1 and auxiliary probe 2 can self-assemble to form DNA concatamers in the solution, the DNA concatamers can not link to the modified electrode. The interaction between the few Ru(phen)3 2+ molecules and the stem-loop structure capture probe only generated a comparatively low ECL background signal (recorded as I 0 ). However, in the presence of target microRNA-21, the DNA probe with stem-loop structure was converted into a rigid, linear double helix DNA with a 15 nucleotide sticky end, which can hybridize with the long DNA concatamers. The intercalation of numerous ECL probe (Ru(phen)3 2+) into the grooves of the DNA concatamers can result in a substantial increase in the corresponding ECL intensity (recorded as I). Using of the DNA nanostructures as carriers for signal amplification, along with the high sensitivity of the ECL technique contribute to the low femtomolar detection of cancer-associated microRNA.
Experimental
Chemicals and reagents
Dichlorotris (1, 10-phenanthro-line) ruthenium hydrate (Ru(phen)3Cl2 · H2O), 6-mercapto-1-hexanol (MCH), Tripropylamine (TPA), disodium ethylene-diaminetetraacetic acid (Na2EDTA) and tris(2-carboxyethyl) phosphine hydrochloride (TCEP) were purchased from Sigma-Aldrich (http://www.sigma-aldrich.com). Other chemicals employed were all of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China, http://www.reagent.com.cn). All solutions were prepared with ultrapure water obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, http://www.millipore.com) with resistivity of 18.2 MΩ · cm. The buffers and solutions involved in this work were as follows: DNA immobilization buffer (I-buffer; 10 mM Tris–HCl + 1 mM EDTA + 1 M NaCl + 10 mM TCEP, pH 7.4), DNA hybridization buffer (H-buffer; 10 mM Tris–HCl + 1 mM EDTA + 300 mM NaCl and 1 mM MgCl2, pH 7.4). Electrochemical impedance spectroscopy and cyclic voltammogram testing buffer (E- buffer 5 mM K4[Fe(CN)6]/K3[Fe(CN)6] containing 0.1 M KCl). 1 × Tris-NaCl-EDTA (TNE) buffer (10 mM Tris–HCl + 0.5 mM EDTA + 10 mM NaCl pH 7.4). All the oligonucleotides used in the present study were synthesized and purified by HPLC by Shanghai Shenggong Biotechnology Co. (Shanghai, China, http://sangon.biogo.net) and used without further purification. The sequences of these oligonucleotides are shown in Table 1.
Apparatus
All electrochemical measurements were carried out on a CHI 660D electrochemical working station (CH Instrument Company, USA, http://www.chinstruments.com). A three-electrode cell (Capacity 10 ml, Diameter 25 mm) consisting of working electrode (the assembled gold electrode, 2 mm in diameter), Ag/AgCl reference electrode and platinum counter electrode was used for all electrochemical measurements. ECL intensity was detected by using a BPCL Ultra-Weak Chemiluminescence Analyzer (http://www.bpcl.com) controlled by a personal computer with BPCL program in conjunction with a CHI 660D electrochemical working station at room temperature. TaqMan MicroRNA Reverse Transcription Kit and TaqMan 2 × Universal PCR Master Mix were purchased from Applied Biosystems (Foster City, CA, http://www.appliedbiosystems.com.cn).
Pretreatment of the gold electrodes
Firstly, after cleaned in piranha solution (30 % H2O2 and 70 % H2SO4 in volume) for 20 min (CAUTION: piranha is a vigorous oxidant and should be used with extreme caution), the gold electrodes were polished sequentially with 0.3 and 0.05 μm alumina slurry for 8 min, followed by ultrasonic cleaning in ethanol and Milli-Q water, respectively. Then, the electrode was washed thoroughly with Milli-Q water and dried in a nitrogen stream to obtain a clean gold surface. The cleanness of the electrode was checked by performing cyclic voltammogram measurement in a fresh 0.5 M H2SO4 solution with the following parameters: potential range from −0.2 to +1.6 V versus Ag/AgCl and scan rate of 0.1 V · s−1. The cleanness continued until a typical single sharp reduction peak located at ∼ 0.88 V and multiple overlapping oxidation peaks in the range of 1.1–1.5 V were clearly observed.
Assembly of DNA sensors
The sensing interface was fabricated as follows: At the beginning of assembly, the cleaned gold electrode was incubated in 6 μL of I-buffer which contained 1 μM thiolated capture probes overnight at room temperature in humidity. The electrode was then thoroughly rinsed with ultrapure water, dried under a stream of nitrogen gas, and incubated in 2 mM MCH solution for 90 min to remove nonspecific DNA adsorption on the gold surface. After rinsed thoroughly, 6 μL of H-buffer containing target microRNA at various concentrations was placed on the modified electrode for 120 min. Then rinsed and dried the electrode. Finally, 10 μL of a freshly prepared H-buffer containing 1 μM auxiliary probe 1 and 1 μM auxiliary probe 2 was dripped on the surface of the electrode, and incubated for 120 min to self-assemble to be DNA concatamers. After rinsed and dried, a droplet of 10 μL 2 mM Ru(phen)3 2+ was added to the modified electrode and incubated for 7 h.
The experimental methods
The electrochemical impedance spectrum (EIS) and cyclic voltammogram (CV) were performed in E-buffer. The ECL intensity of the resulting functionalized electrode was recorded in phosphate buffer solution (0.1 M, pH 7.5) containing 20 mM TPA from 0 to 1.35 V (versus Ag/AgCl) at the scan rate of 50 mV · s-1. The voltage of the photomultiplier tube (PMT) was set at 800 V in the process of detection.
Results and discussion
Characterization of the immobilization process of the modified gold electrode
The EIS and CV of ferricyanide are valuable and convenient tools to monitor the surface status and the barrier of the modified electrode. Therefore, EIS and CV measurements of different modified electrodes in the presence of a 5 mM K4[Fe(CN)6]/K3[Fe(CN)6] mixture as a redox probe are shown in Figure 2. Figure 2a shows the EIS at different modified electrodes in the impedance spectra, the increase in semicircle diameter indicates the increase in the electron transfer resistance (Ret) at the interface [32]. It can be seen that the bare electrode exhibits only a tiny semicircle domain, which implies fast electron transfer process. When capture probe was self-assembled onto the gold electrode, the Ret increased significantly compared with the bare gold electrode. This is attributed to the negative charges on the phosphate backbone of the self-assembled capture probe DNA, which produce an electrostatic repulsive force to Fe(CN)6 4−/3−. The Ret further increased upon the hybridization of the target microRNA and AP1, (curve c, d). After the formation of long DNA concatamers, electron transfer became more difficult, and the Ret was further dramatically enlarged (curve e). The results were consistent with the observation from CV as shown in Fig. 2b. The results of CV and EIS both confirmed the successful capture of the target microRNA-21 and the formation of long DNA concatamers.
a Electrochemical impedance spectrum measurements of different modified electrodes in the presence of 0.1 M KCl containing a 5 mM K4[Fe(CN)6]/K3[Fe(CN)6] mixture and b the cyclic voltammogram: (a) bare GE, (b) a capture probe modified GE, (c) target microRNA/MCH/capture probe modified GE, (d) AP1/target microRNA/MCH/capture probe modified GE, (e) AP1 + AP2/target microRNA/MCH/capture probe modified GE
Performance of the biosensor
The ECL emission signals of the biosensor were measured after the modified electrode were immersed in 20 mM TPA containing 0.1 M phosphate buffer solution (pH 7.5) and exerted a sweep potential from 0 to 1.35 V. We have investigated the changes between I and I 0 (recorded as △I, △I = I-I 0 ), when a series of different concentrations of microRNA-21 was incubated. As shown in Fig. 3, it is clear that the addition of target microRNA-21 at different concentrations to the sensor induced different increases in the ECL signals. The increase in ECL emissions was linear with the logarithm of microRNA-21 concentrations. The liner range for the target microRNA is from 1 fM to 10 pM with a real detection limit of 1 fM. The linear equation was △I =2222.5 log C + 36013 with a linear correlation of R2 = 0.9938, C is the concentration of target microRNA-21. In addition, the relative standard deviation (RSD, n = 3) of the target microRNA with different electrodes from the same batch at 1 pM level was 7.2 %, indicating the good reproducibility of the ECL biosensor.
ECL responses of the sensor to different concentration of target microRNA (a) 0 fM, (b) 1 fM, (c) 10 fM, (d) 100 fM, (e) 1 pM, (f) 2.5 pM, (g) 10 pM. Inset: The resulting calibration plot of log c vs ECL intensity (△I). The illustrated error bars represent the standard deviation of three repetitive measurements
Here, we consider that the ultrasensitivity comes from the significant length of the self-assembled DNA nanostructures. Our previous studies confirmed that compared with other carriers for signal amplification, the DNA nanostructures with the length in micrometers can accumulate many more indicators and produce much larger signal [25, 33]. Therefore, numerous Ru(phen)3 2+ molecules intercalating into the grooves of the fabricated long-range self-assembled DNA concatamer with high affinity can result in tremendously amplified ECL signal output.
The selectivity of the biosensor
The biosensor also shows high selectivity for microRNA-21 detection. In this work, four kinds of microRNA sequences including complementary target microRNA, single-base mismatch target, two-base mismatch target, and noncomplementary sequence were chosen to study the selectivity of the biosensor. The concentration of TR was 10 pM, while the concentrations of three other microRNA sequences were 1 nM. Despite of the large concentrations of disrupting microRNA, the change in ECL response induced by the non-specific hybridization between the interference above was much lower than that of the response induced by the specific hybridization of microRNA-21 (Fig. 4a-e), suggesting that the fabricated label-free and ultrasensitive electrochemiluminescence biosensor was able to discriminate the target microRNA with high specificity and sensitivity.
Selectivity investigation for the blank and the four various RNA sequences: (a) blank, (b) noncomplementary sequence (NC), (c) two-base mismatch target (2MT), (d) single-base mismatch targe (1MT), (e) target microRNA-21. The concentrations of 1MT, 2MT, and NC were 1 nM. The concentration of target microRNA-21 was 10 pM
Comparison between the method and quantitative real-time polymerase chain reaction
Since microRNA-21 is a potential cancer biomarker which has been identified with elevated expression levels in numerous tumor tissues, such as breast, diffuse large B-cell lymphoma, ovarian cancer and myocardial diseases, we used the fabricated biosensor to quantify the expressing of microRNA-21 in MCF-7 cells (Invasive breast ductal carcinoma cell line). The total RNA of MCF-7 cells were purchased from Genetimes Technology, Inc. (http://genetimes.bioon.com.cn). The sample was diluted with TNE buffer and used for detection. For the purpose of result validation, we quantified the content of microRNA-21 in the same batch of cells by quantitative real-time polymerase chain reaction (qRT-PCR) method. The results are shown in Figure 5. The result of the presented method agrees well with the outcome of the qRT-PCR method, which demonstrates that the biosensor developed here is of high potential in profiling expression of microRNAs in samples containing many different-sequence RNAs. Thus, this ultrasensitive electrochemiluminescence biosensor may be applicable to early cancer diagnosis.
Conclusions
In conclusion, we have demonstrated the construction of a simple and highly sensitive ECL biosensor for detecting microRNA-21 at the low femtomolar level based on long-range self-assembled DNA nanostructures. The ECL-based protocol has apparent advantages. Firstly, this biosensor can detect as low as 1 fM target microRNA, with sensitivity similar to that of PCR. Secondly, this ECL biosensor is really simple and inexpensive, while the chemical labeling of the DNA strand and the preparation of nanomaterials are not required. Thirdly, the fabricated biosensor has been used for quantifying target microRNA in cancerous cell lines. Therefore, the simple ultrasensitive ECL protocol can be built to provide an alternative approach for microRNA detection in diagnostics and gene analysis.
References
Dai H, Wang YM, Wu XP, Zhang L, Chen GN (2009) An electrochemiluminescent sensor for methamphetamine hydrochloride based on multiwall carbon nanotube/ionic liquid composite electrode. Biosens Bioelectron 24:1230–1234
Li JX, Yang LX, Luo SL, Chen BB, Li J, Lin HL, Cai QY, Yao SZ (2010) Polycyclic aromatic hydrocarbon detection by electrochemiluminescence generating Ag/TiO2 nanotubes. Anal Chem 82:7357–7361
Tang CX, Zhao Y, He XW, Yin XB (2010) A “turn-on” electrochemiluminescent biosensor for detecting Hg2+ at femtomole level based on the intercalation of Ru(phen)3 2+ into ds-DNA. Chem Commun 46:9022–9024
Yuan T, Liu ZY, Hu LZ, Zhang L, Xu GB (2011) Label-free supersandwich electrochemiluminescence assay for detection of sub-nanomolar Hg2+. Chem Commun 47:11951–11953
Gao A, Tang CX, He XW, Yin XB (2013) Electrochemiluminescent lead biosensor based on GR-5 lead-dependent DNAzyme for Ru(phen)3 2+ intercalation and lead recognition. Analyst 138:263–268
Ding CF, Zheng Q, Wang NN, Yue QF (2012) An electrochemiluminescence strategy based on aptamers and nanoparticles for the detection of cancer cells. Anal Chim Acta 756:73–78
Yao W, Wang L, Wang HY, Zhang XL (2009) Electrochemiluminescent sensor for the detection of DNA hybridization using stem-loop structure DNA as capture probes. Microchim Acta 165:407–413
Yao W, Wang L, Wang HY, Zhang XL, Li L, Zhang N, Pan L, Xing NN (2013) An electrochemiluminescent DNA sensor based on nano-gold enhancement and ferrocene quenching. Biosens Bioelectron 40:356–361
Yin XB, Xin YY, Zhao Y (2009) Label-Free electrochemiluminescent aptasensor with attomolar mass detection limits based on a Ru(phen)3 2+-double-strand DNA composite film electrode. Anal Chem 81:9299–9305
Jie G, Yuan JX (2012) Novel magnetic Fe3O4@CdSe composite quantum dot-based electrochemiluminescence detection of thrombin by a multiple DNA cycle amplification strategy. Anal Chem 84:2811–2817
Miao WJ (2008) Electrogenerated chemiluminescence and its biorelated applications. Chem Rev 108:2506–2553
Zhan W, Bard AJ (2007) Electrogenerated chemiluminescence. 83. immunoassay of human C-Reactive protein by using Ru(bpy)3 2+-encapsulated liposomes as labels. Anal Chem 79:459–463
Wang H, Zhang CX, Li Y, Qi HL (2006) Electrogenerated chemiluminescence detection for deoxyribonucleic acid hybridization based on gold nanoparticles carrying multiple probes. Anal Chim Acta 575:205–211
Chai Y, Tian DY, Wang W, Cui H (2010) A novel electrochemiluminescence strategy for ultrasensitive DNA assay using luminol functionalized gold nanoparticles multi-labeling and amplification of gold nanoparticles and biotin-streptavidin system. Chem Commun 46:7560–7562
Li Y, Qi HL, Fang F, Zhang CX (2007) Ultrasensitive electrogenerated chemiluminescence detection of DNA hybridization using carbon-nanotubes loaded with tris(2,2″-bipyridyl) ruthenium derivative tags. Talanta 72:1704–1709
Naimish PS, John CB, James FR (2011) Carbon nanotube microwell array for sensitive electrochemiluminescent detection of cancer biomarker proteins. Anal Chem 83:6698–6703
Chu HH, Yan JL, Tu YF (2011) Electrochemiluminescent detection of the hybridization of oligonucleotides using an electrode modified with nanocomposite of carbon nanotubes and gold nanoparticles. Microchim Acta 175:209–216
Wei H, Liu JF, Zhou LL, Li J, Jiang X, Kang JZ, Yang XR, Dong SJ, Wang EK (2008) [Ru(bpy)3]2+-doped silica nanoparticles within layer-by-layer biomolecular coatings and their application as a biocompatible electrochemiluminescent tag material. Chem Eur J 14:3687–3693
Sun QX, Zou GZ, Zhang XL (2011) Electrochemiluminescence DNA sensor based on hairpin structure DNA as recognition element and Ru(bpy)3 2+-doped silica nanoparticles as signal-producing compound. Electroanalysis 23:2693–2698
Seeman NCJ (1982) Nucleic acid junctions and lattices. Theor Biol 99:237–247
Seeman NCJ (2010) Nanomaterials Based on DNA. Annu Rev Biochem 79:65–87
Teller C, Willner I (2010) Organizing protein-DNA hybrids as nanostructures with programmed functionalities. Trends Biotechnol 28:619–628
Pinheiro AV, Han DR, Shih WM, Yan H (2011) Challenges and opportunities for structural DNA nanotechnology. Nat Nanotechnol 6:763–772
Pei H, Lu N, Wen YL, Song SP, Liu Y, Yan H, Fan CH (2010) A DNA Nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv Mater 22:4754–4758
Chen X, Lin YH, Li J, Lin LS, Chen GN, Yang HH (2011) A simple and ultrasensitive electrochemical DNA biosensor based on DNA concatamers. Chem Commun 47:12116–12118
Shimron S, Wang FA, Orbach R, Willner I (2012) Amplified detection of DNA through the enzyme-free autonomous assembly of hemin/G-quadruplex DNAzyme nanowires. Anal Chem 84:1042–1048
Dirks RM, Pierce NA (2004) Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A 101:15275–15278
Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L (2011) Intracellular and Extracellular MicroRNAs in breast cancer. Clin Chem 57:18–32
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CSR, Harris AL (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141:672–67530
Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE (2009) The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112:55–59
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JTR, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456:980–984
Zhang SS, Xia JP, Li XM (2008) Electrochemical biosensor for detection of adenosine based on structure-switching aptamer and amplification with reporter probe DNA modified Au nanoparticles. Anal Chem 80:8382–8388
Chen X, Hong CY, Lin YH, Chen JH, Chen GN, Yang HH (2012) Enzyme-free and label-free ultrasensitive electrochemical detection of human immunodeficiency virus DNA in biological samples based on long-range self-assembled DNA nanostructures. Anal Chem 84:8277–8283
Acknowledgments
The authors gratefully acknowledge the financial support of the National Basic Research Program of China (No. 2010CB732403), the National Natural Science Foundation of China (No. 21125524, No. 21105012), the Program For New Century Excellent Talents in University of China (09-0014) and the National Science Foundation of Fujian Province (2010 J06003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, T., Chen, X., Hong, CY. et al. Label-free and ultrasensitive electrochemiluminescence detection of microRNA based on long-range self-assembled DNA nanostructures. Microchim Acta 181, 731–736 (2014). https://doi.org/10.1007/s00604-013-1113-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1113-5