Abstract
We report on a competitive immunoassay for the determination of aflatoxin B1 using fluorescence resonance energy transfer (FRET) from anti-aflatoxin B1 antibody (immobilized on the shell of CdTe quantum dots) to Rhodamine 123 (Rho 123-labeled aflatoxin B1 bound to albumin). The highly specific immunoreaction between the antibody against aflatoxin B1 on the QDs and the labeled-aflatoxin B1 brings the Rho 123 fluorophore (acting as the acceptor) and the QDs (acting as the donor) in close spatial proximity and causes FRET to occur upon photoexcitation of the QDs. In the absence of unlabeled aflatoxin B1, the antigen-antibody complex is stable, and strong emission resulting from the FRET from QDs to labeled aflatoxin B1 is observed. In the presence of aflatoxin B1, it will compete with the labeled aflatoxin B1-albumin complex for binding to the antibody-QDs conjugate so that FRET will be increasingly suppressed. The reduction in the fluorescence intensity of the acceptor correlates well with the concentration of aflatoxin B1. The feasibility of the method was established by the detection of aflatoxin B1 in spiked human serum. There is a linear relationship between the increased fluorescence intensity of Rho 123 with increasing concentration of aflatoxin B1 in spike human serum, over the range of 0.1–0.6 μmol·mL−1. The limit of detection is 2 × 10−11 M. This homogeneous competitive detection scheme is simple, rapid and efficient, and does not require excessive washing and separation steps.

A nanobiosensor has been fabricated based on a competitive immunoassay for the determination of aflatoxin B1 using fluorescence resonance energy transfer (FRET). In the presence of aflatoxin B1, it will compete with the labeled aflatoxin B1-albumin complex for binding to the antibody-QDs conjugate so that FRET will be increasingly suppressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins are a group of potent hepatotoxic, mutagenic, carcinogenic, immunosuppressive secondary metabolites produced mainly by the fungi of Aspergillus flavus and A. parasiticus and are considered highly harmful to human, poultry and livestock health [1, 2]. Under favorable growth circumstances for these toxigenous fungi, aflatoxins maybe produced during production, transfer and storage of food products [3]. Aflatoxin B1, the most common and most toxic of the aflatoxins, is the focus of governments and researchers all over the world due to the potent hepatotoxic and carcinogenic effects revealed in animal studies. Thus, there are reasonable economic and safety reasons for establishing highly sensitive, selective, cost-effective and rapid analytical methods for regular screening of aflatoxin B1 in a variety of specimens [4]. To date, several methods such as high performance liquid chromatography, thin layer chromatography, as well as enzyme-linked immunosorbent assays have been used for detection of aflatoxin B1 in different specimens. Within the last few years, some new nanobiosensors using different nanoparticles or microarray systems for detection of mycotoxins have also been designed [5–10]. However, there is still a need for more rapid, sensitive systems capable of early detection of aflatoxicosis in order to protect individuals exposed to high-risk environments and conditions. In recent years, quantum dots (QDs) due to their unique characteristics e.g. easy handling, and being ideal donors have been extensively studied in fluorescence resonance energy transfer (FRET)-based nanobiosensor for rapid and highly-sensitive detection and diagnosis of different kinds of molecules and diseases [11, 12]. QDs are composed of substances among II–VI and III–V semiconductor elements, which possess smaller sizes than the exciton Bohr radius [13]. In fact, they are highly efficient fluorophores with a strong band gap luminescence tuned by their size, narrow symmetric bandwidth emission spectra (tunable by their sizes, compositions, and shapes) and high resistance to photo-bleaching, allowing for prolonged observation and multiplexing [14, 15]. These unique optical properties have made QDs one of the most important labeling materials for biological and medical diagnostic applications.
QDs are approximately spherical, the shell of which can be capped with various water-soluble functional groups, and are therefore easily dispersed in water [16, 17]. These surface functional groups could attach to biomolecules to form QD-nanobioconjugate. An example for this case is the terminal carboxylic groups linked to various biomolecules [18, 19]. As mentioned earlier, QDs as ideal donors possess many advantages in FRET applications [20, 21]. More specifically, FRET occurs when the electronic excitation energy of a donor fluorophore is transferred to a nearby acceptor molecule via a through-space dipole–dipole interaction between the donor–acceptor pair [22]. Based on Förster’s theory, the rate of energy transfer depends mainly on the following factors [10]; (1) the extent of spectral overlap between the donor emission wavelength and the acceptor UV–Vis absorption wavelength, (2) the quantum yield of the donor, (3) the relative orientation of the donor and acceptor transition dipoles, and (4) the distance between the donor and acceptor transition dipoles.
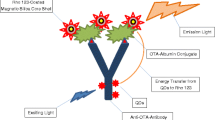
The present study was set to develop a very high sensitive, simple and rapid FRET-based nanobiosensor to detect aflatoxin B1 (Scheme 1). Rhodamine 123 (Rho 123) and QDs were used as acceptor and donor, respectively. The QDs-labeled anti-aflatoxin B1 antibody and Rho 123-labeled aflatoxin B1-albumin constructed the two moieties of the nanobiosensor. In fact, the Rho 123-labeled aflatoxin B1-albumin formed an immuno-complex with the QDs-labeled anti-aflatoxin B1 antibody. The proximity of the QDs and the Rho 123 resulted in facilitated energy transfer from the donor to the acceptor. Hence, maximum fluorescence intensity of Rho 123 was observed upon photo-excitation of the QDs. In the presence of aflatoxin B1 in a specimen, the labeled-aflatoxin B1 was competitively replaced by aflatoxin B1 that led to reduction of the fluorescence intensity of Rho 123.
Schematic representation of the designed nanobiosensor for detection of aflatoxin B1 based on a FRET phenomenon using QDs-anti-aflatoxin B1 antibody as a donor and Rho 123-labeled albumin-aflatoxin B1 as an acceptor. Fluorescence of the QDs was observed at 505 nm, when exited at 375 nm. In the presence of aflatoxin B1 in the nanobiosensor, significant reduction in the emission intensity was observed
Experimental
Materials and apparatus
Aflatoxin B1, aflatoxin B1-albumin conjugate, anti-aflatoxin B1 antibody, cadmium chloride (CdCl2), sodium borohydride (NaBH4), tellurium powder (Te), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) were purchased from Sigma chemical company (St. Louis, Mo, http://www.sigmaaldrich.com). Thioglycolic acid (TGA) and all other chemicals were bought from Merck chemical company (Darmstadt, Germany, http://www.merck.de). All the materials were used as supplied without further purification and all the solutions were prepared using double-distilled water.
All fluorescence spectra were recorded on a Shimadzu fluorescence spectrometer (Japan, http://www.shimadzu.com). All optical measurements were carried out under ambient conditions. The transmission electron microscopy (TEM) image of the prepared QDs was acquired on an H-600; Philips (Netherlands, http://www.philips.com). A Malvern dynamic light scattering (DLS) apparatus (UK, http://www.Malvern.com) was used to investigate the size distribution of the synthesized QDs. All the experiments were carried out in triplicate.
Preparation of TGA-capped CdTe QDs
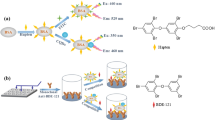
The water-soluble CdTe QDs were synthesized and characterized according to the procedure reported in our previous work [12, 23, 24]. Briefly, 0.1 g Te powder was reduced by 0.280 g NaBH4 in 7 mL double-distilled water under stirring and N2 bubbling. After 5 h, color of the solution changed from violet to white. The solution was ultra-filtered under nitrogen atmosphere to remove white precipitate of sodium tetraborate. Then the fresh prepared oxygen-free NaHTe aqueous solution was added into a 0.358 g CdCl2 2.5 H2O in 200 mL nitrogen-saturated double-distilled water at pH 10.0 in the presence of 0.2 mL TGA as a stabilizing and capping agent. The mixture was refluxed under a nitrogen atmosphere while vigorously stirring. QDs with different sizes could be obtained by prolonging the refluxing time. For removing excess precursors, the crude solution was washed three times with ethanol and centrifuged for 15 min at 10,000 × g. The resulting precipitate was re-dispersed in 250 mL water and kept at 4 °C in dark. Maximum excitation and emission wavelength were at 375 and 505 nm, respectively.
Preparation of QDs-anti-aflatoxin B1 antibody nanobioconjugate
For preparation of QD-anti-aflatoxin B1 antibody nanobioconjugate, 1 mL of the prepared QDs solution was mixed with 5 mL of solution of EDC (1 mg) and NHS (1 mg) at pH 6.0 and incubated for 1 h at room temperature in a dark place. Subsequently, the ice-cold solution of anti-aflatoxin B1 antibody was added by drop wise addition to the ice-cold EDC/NHS activated QDs solution while gently stirring, and the whole solution was kept for 1 h in this condition in dark. In order to characterize the formation of the QDs-anti-aflatoxin B1 antibody nanobioconjugate, optical density of the sample of anti-aflatoxin B1 antibody and QDs-anti-aflatoxin B1 antibody nanobioconjugate at 280 nm, before and after centrifugation at 10,000 × g for 20 min at 4 °C, were recorded on a spectrophotometer. The stock solution was kept at 4 °C in dark for further use.
Labeling of aflatoxin B1-albumin with Rho 123
For preparation of Rho 123-labeled aflatoxin B1-albumin bioconjugate, 1 mg of Rho 123 was dissolved in 1 mL of phosphate buffer solution (50 mM, pH 6) and was then chilled. The prepared solution was added to 2 mL of ice-cold phosphate buffer solution (50 mM, pH 6) containing 1 mg of aflatoxin B1-albumin. The homogenized mixture was prepared by repeated and gently pipetting. One mL of solution of EDC (1 mg) and NHS (1 mg) at pH 6.0 was then added to the prepared mixture and incubated for 1 h at 4 °C in dark. Consequently, the Rho 123-labeled aflatoxin B1-albumin was dialyzed (cutoff: 10 kDa) against 2 × 1 L of 50 mM phosphate buffer pH 7.4 to remove excess substances. In order to characterize the labeling of the aflatoxin B1-albumin with Rho 123, optical density of the dialyzed mixture was measured on a spectrophotometer at 450 nm. The aflatoxin B1-albumin solution was considered as a blank. Because of photosensitivity of Rho 123, the stock solution was kept at 4 °C in dark for further use.
Biosensor optimization and evaluation
In order to optimize the ratio between QDs-labeled anti-aflatoxin B1 antibody (conjugate 1), and the Rho 123-labeled aflatoxin B1-albumin (conjugate 2), varying concentrations of conjugate 2 (0.1 to 1 μM; 0.1 μM intervals), were sequentially added to a constant concentration of conjugate 1 (0.3 μM). The optimal concentration of conjugate 2 was then determined as the point where the emission of Rho 123 reached a constant value. In fact, all the antigen-binding sites of the anti-aflatoxin B1 antibody were saturated at that point.
For evaluation of the ability of the designed nanobiosensor for detecting aflatoxin B1, a reaction mixture containing the previously measured optimal value of Rho 123-labeled aflatoxin B1-albumin bioconjugate (0.6 μM), and 0.3 μM QDs-labeled anti-aflatoxin B1 antibody in 50 mM sodium phosphate buffer (pH 7.4) and 100 mM NaCl was prepared. After pipeting and incubating the reaction for 3 min to ensure the formation of immuno-complex of the Rho 123-labeled aflatoxin B1-albumin bioconjugate and the QDs-labeled anti-aflatoxin B1 antibody, the samples were excited at 375 nm and emission spectra (450–620 nm) were recorded on a spectrofluorimeter. Excitation and emission bandwidth were selected at 10 nm and 15 nm, respectively. A stock solution of 1 mg.mL−1 of aflatoxin B1 in 50 mM sodium phosphate buffer (pH 7.4) and 100 mM NaCl was prepared and was then sequentially added to the reaction mixture. Emission spectra of the samples were recorded after incubating the reaction mixture for 3 min at room temperature in a 1 cm pass-length quartz cell, at the same condition as mentioned above.
Results
Characterization of the prepared CdTe QDs
Absorption and emission spectra of the pure solution of the prepared CdTe QDs and Rho 123 are shown in Fig. 1. As seen, the results obtained showed the maximum emission peak of the QDs at 505 nm, while the maximum absorption and emission peaks of Rho 123 were recorded at 510 nm and 580 nm, respectively. The full width at half maximum (FWHM) of the QDs emission spectrum was at about 35 nm. Emission wavelength of the QDs and absorption wavelength of Rho 123 showed maximum spectral overlap that is critical for obtaining optimum FRET phenomena. As shown in Fig. 2, the TEM image and DLS analysis of the prepared TGA-capped CdTe QDs revealed that, the core/shell had a spherical morphology with a particle size of about 3 nm as well as good monodispersity. Moreover, the obtained QDs dispersed in the phosphate buffer (pH 7.4) showed high optical stability, without considerable loss of fluorescence intensity during 3 months (data not shown).
Characterization of the QDs-anti-aflatoxin B1 antibody nanobioconjugate
Reduction in optical density of the QDs-anti-aflatoxin B1 antibody nanobioconjugate solution after high-speed centrifugation at 280 nm confirmed successful covalent coupling between the anti-aflatoxin B1 antibody and the QDs. Immobilization of the anti-aflatoxin B1 antibody on the shell of the prepared QDs did not alter their emission spectra.
Labeling of aflatoxin B1-albumin with Rho 123
To verify the labeling of the aflatoxin B1-albumin with Rho 123, optical density of the dialyzed mixture of aflatoxin B1-albumin-Rho 123 was measured at Rho 123’s maximal absorbance wavelength of 510 nm on a spectrophotometer. A strong absorption at 510 nm showed the presence of Rho 123 in the sample.
Energy transfer between QDs and Rho 123
The FRET system could be obviously observed, when the immunoreaction occurred between the QDs-labeled anti-aflatoxin B1 antibody and Rho 123-labeled aflatoxin B1-albumin. The fluorescence intensity of Rho 123 at 580 nm was enhanced upon sequential addition (0.1 μM intervals) of a solution containing Rho 123-labeled aflatoxin B1-albumin, from 0.1 μM to 0.6 μM where the maximum fluorescence intensity was obtained. The observed changes in Rho 123 emission were directly proportional to the final concentration of the Rho 123-labeled aflatoxin B1-albumin until all the antigen binding capacity of the anti-aflatoxin B1 antibody were occupied with the Rho 123-labeled aflatoxin B1-albumin. However, a further increase in the concentration of the Rho 123-labeled aflatoxin B1-albumin resulted in no significant enhancement in the emission peak of Rho 123. By adding a specimen containing aflatoxin B1, the Rho 123-labeled aflatoxin B1-albumin could be competitively replaced by aflatoxin B1 that led to reduction in the fluorescence intensity of the system (Fig. 3). Figure 4 clearly indicates the relations between the decreased fluorescence intensity of Rho 123 at 580 nm and increased fluorescence intensity of QDs at 505 nm with the increasing concentrations of aflatoxin B1 in specimen, over the range of 0.1 μM to 0.6 μM. The limit of detection (LOD) was estimated at 2 × 10−11 M, based on the equation LOD = 3S0. K− 1, where S0 is the standard deviation of blank measurements (n = 7) and K is the slope of calibration curve.
Changes in fluorescence intensity of the QDs and Rho 123 in the designed nanobiosensor. Fluorescence intensity of Rho 123 sequentially decreased when different volumes of the aflatoxin B1-spiked specimen was added to the reaction mixture. The upward-arrow indicates the increasing emission of QDs while the downward arrow indicates the decreasing emission of Rho 123, caused by the addition of label-free aflatoxin B1 (contained in the sample)
Discussion
In this work, we prepared a FRET-based nonobiosensor for detection of aflatoxin B1 using the anti-aflatoxin B1 antibody, aflatoxin B1-albumin conjugate, Rho 123 and CdTe QDs core/shell. QDs-Rho 123 FRET-based nanobiosensors have been previously used in detecting a number of pathogens such as Candidatus Phytoplasma aurantifolia; the causative agent of witches broom disease of lime [12] and Helicobacter pylori, the cause of gastric ulcer [24]. However, to the best of our knowledge, this was the first attempt to develop such nanobiosensor for detection of aflatoxin B1.
More specifically, water-soluble, highly monodispersed and stable CdTe QDs were synthesized to fabricate the nanobiosensor. Characterization of the synthesized QDs by spectrophotometer, spectrofluorimeter, TEM and DLS confirmed that the particles were suitable for the designed nonobiosensor. Since the excitation wavelength of Rho 123 was within the emission spectra of the synthesized QDs, they were selected as an acceptor and donor, respectively. In fact, it is well feasible to choose an extended excitation wavelength due to the wide absorption spectrum of QDs. On other hand, as Rho 123-labeled aflatoxin B1-albumin showed no significant fluorescence intensity at the emission wavelength of 375 nm, it was selected as the excitation wavelength for the FRET system.
To combine the QDs with anti-aflatoxin B1 antibody, the QDs were attached to the anti-aflatoxin B1 antibody via covalent interactions between carboxyl groups of the TGA-capped QDs and amino groups of the antibody. To obtain maximal FRET signal between QDs and Rho 123, different concentrations of Rho 123-labeled aflatoxin B1-albumin and QDs-labeled anti-aflatoxin B1 antibody were tested for the nanobiosensor preparation. Optimum immunoreaction between the Rho 123-labeled aflatoxin B1-albumin and the QDs-labeled anti-aflatoxin B1 antibody was achieved at a molar ratio of 0.6:03 μM, respectively. At this ratio, the immunoreaction of two molecules of aflatoxin B1 with one molecule of antibody is predictable.
More specifically, at lower molar ratios, excess QDs-labeled anti-aflatoxin B1 antibody which did not participate in the formation of the aflatoxin B1-antibody immuno-complex reacts with free aflatoxin B1. This would particularly jeopardize the detection system at lower concentrations of aflatoxin B1 in specimen where no significant changes in FRET signals occur. In another words, because of the existence of the free antibody, which in turn binds to aflatoxin B1 in specimen, the concentration of aflatoxin B1 in specimen is underestimated.
At higher molar ratios, excess of the Rho 123-labeled aflatoxin B1 leads to loss of accuracy and sensitivity of the nanobiosensor. In other words, due to the presence of the excess Rho 123-labeled aflatoxin B1-albumin, higher concentrations of aflatoxin B1 are required to overcome competitive replacement in binding sites of the anti-aflatoxin B1 antibody. This leads to loss of the accuracy and the sensitivity of the nanobiosensor especially in the detection of lower concentrations of aflatoxin B1 in specimen. As mentioned and summarized in Table 1 and in comparison with the similar investigations, the detection limit obtained in this study (2 × 10−11 M) was very close to those reported previously.
Overall, since anti-aflatoxin B1 antibody and aflatoxin B1-albumin were labeled with many QDs and Rho 123, respectively, a considerable energy transfer between the QDs and the Rho 123 was achieved which led to high sensitivity of the designed nanobiosensor. Therefore and based on the results obtained herein, it was possible to estimate even very low concentrations of aflatoxin B1 in patient’s specimens or agricultural products by comparing the obtained signals with a standard curve. Finally, although fluorophores such as Rho 123 could also be used in labeling antigens or antibodies in heterogeneous fluorescence-based immunoassays but unlike nano-fluorescence-based immunoassays, such systems are time-consuming and not comparatively easy to handle as they require multiple reagent addition and washing steps. Moreover, nanobiosensors are easy to be automated. Therefore, the designed nanobiosensor was found to be so efficient, simple, rapid and homogeneous that does not require excessive washing and separation steps.
Conclusion
QDs-anti-aflatoxin B1 antibody and Rho 123-aflatoxin B1-albumin conjugates, as fluorescence probes were prepared to detect aflatoxin B1 based on the FRET phenomenon. In fact, the close proximity of the QDs core/shell and Rho 123 molecules in immuno-complex between the QDs-labeled anti-aflatoxin B1 antibody and the Rho 123-labeled aflatoxin B1-albumin resulted in FRET phenomenon. By adding aflatoxin B1-contaminated sample, the Rho 123-labeled aflatoxin B1-albumin was replaced by aflatoxin B1 and by further addition of the sample, the fluorescence intensity of the system was reduced to background signal. We strongly believe that through further investigation, this simple, rapid and highly sensitive FRET system could be used in detecting different microbial toxins.
References
Devi KT, Mayo MA, Reddy KL, Delfosse P, Reddy G, Reddy SV, Reddy DV (1999) Production and characterization of monoclonal antibodies for aflatoxin B1. Lett Appl Microbiol 29:284
Hussein HS, Brasel JM (2001) Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicol 167:101
Kamkar A, Jahed Khaniki GR, Alavi AS (2011) Occurrence of aflatoxin M1 in raw milk produced in Ardabil of Iran. Iran J Environ Health Sci Eng 8:123–128
Oldano F, Ruffier M, Sezian A, Bandirola C (2007) Aflatoxins occurrence in milk and feed in Northern Italy during 2004–2005. Food Control 18:1263
Sapsford KE, Taitt CR, Fertig S, Moore MH, Lassman ME, Maragos CM, Shriver-Lake LC (2006) Indirect competitive immunoassay for detection of aflatoxin B1 in corn and nut products using the array biosensor. Biosens Bioelectron 21:2298
Liao JY, Li H (2010) Lateral flow immunodipstick for visual detection of aflatoxin B1 in food using immuno-nanoparticles composed of a silver core and a gold shell. Microchim Acta 171:289
Xiulan S, Xiaolian Z, Jian T, Xiaohong G, Jun Z, Chu FS (2006) Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int J Food Microbiol 99:185
Xiulan S, Xiaolian Z, Jian T, Xiaohong G, Jun Z, Chu FS (2006) Development of an immunochromatographic assay for detection of aflatoxin B1 in foods. Food Control 17:256
Lamberti I, Tanzarella C, Solinas I, Padula C, Mosiello L (2009) An antibody-based microarray assay for the simultaneous detection of aflatoxin B1 and fumonisin B1. Mycotox Res 25:193
Bacher G, Pal S, Kanungo L, Bhand S (2012) A label-free silver wire based impedimetric immunosensor for detection of aflatoxin M1 in milk. Sensors Actuators B Chem 128:223
Dong F, Kewang H, Han H, Liang J (2009) A novel method for methimazole determination using CdSe quantum dots as fluorescence probes. Microchim Acta 165:195
Rad F, Mohsenifar A, Tabatabaei M, Safarnejad MR, Sahryari F, Safarpour H, Foroutan A, Mardi M, Davoudi D, Fotokian M (2012) Detection of candidatus phytoplasma aurantifolia with a quantum dots FRET-based biosensor. J Plant Pathol 94:525
Costa-Fernandez JM (2006) Optical sensors based on luminescent quantum dots. Anal Bioanal Chem 384:37
Clapp AR, Medintz IL, Mattoussi H (2006) Förster resonance energy transfer investigations using quantum-dot fluorophores. Chemphyschem 16:47
Zhong P, He G, Zhang M (2012) Optimal spectra of white light-emitting diodes using quantum dot nanophosphors. Opt Express 20:9122
Zhang H, Liu L, Fu X, Zhu Z (2012) Microfluidic beads-based immunosensor for sensitive detection of cancer biomarker proteins using multienzyme-nanoparticle amplification and quantum dots labels. Biosens Bioelectron 42:23
Jaiswal JK, Goldman ER, Mattoussi H, Simon SM (2004) Use of quantum dots for live cell imaging. Nat Methods 1:73
Medintz IL, Konnert JH, Clapp AR, Stanish I, Twigg ME, Mattoussi H, Mauro JM, Deschamps JR (2004) A fluorescence resonance energy transfer-derived structure of a quantum dot-protein bioconjugate nanoassembly. Proc Natl Acad Sci U S A 101:9612
Goldman ER, Balighian ED, Mattoussi H, Kuno MK, Mauro JM, Tran PT, Anderson GP (2002) Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc 124:6378
Algar WR, Malanoski AP, Susumu K, Stewart MH, Hildebrandt N, Medintz IL (2012) Multiplexed tracking of protease activity using a single color of quantum dot vector and a time-gated förster resonance energy transfer relay. Anal Chem 84:10136
Pei J, Zhu H, Wang X, Zhang H, Yang X (2012) Synthesis of cysteamine-coated CdTe quantum dots and its application in mercury (II) detection. Anal Chim Acta 757:63
Medintz IL, Mattoussi H (2009) Quantum dot-based resonance energy transfer and its growing application in biology. Phys Chem Chem Phys 11:17
Shamsipur M, Shanehasz M, Khajeh K, Mollania N, Kazemi SH (2012) A novel quantum dot-laccase hybrid nanobiosensor for low level determination of dopamine. Analyst 137:5553
Shanehsaz M, Mohsenifar A, Hasannia S, Pirooznia N, Samaei Y, Shamsipur M (2013) Detection of Helicobacter pylori with a anobiosensor based on fluorescence resonance energy transfer using CdTe quantum dots. Microchim Acta 180:195
Acknowledgments
We appreciate the financial support provided by Nanozino Company. The authors would like to thank Mrs. Batool Etemadikia for her valuable technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zekavati, R., Safi, S., Hashemi, S.J. et al. Highly sensitive FRET-based fluorescence immunoassay for aflatoxin B1 using cadmium telluride quantum dots. Microchim Acta 180, 1217–1223 (2013). https://doi.org/10.1007/s00604-013-1047-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1047-y