Abstract
We report on a sensitive electrochemical sensor for dopamine (DA) based on a glassy carbon electrode that was modified with a nanocomposite containing electrochemically reduced graphene oxide (RGO) and palladium nanoparticles (Pd-NPs). The composite was characterized by scanning electron microscopy, energy dispersive spectroscopy, and electrochemical impendence spectroscopy. The electrode can oxidize DA at lower potential (234 mV vs Ag/AgCl) than electrodes modified with RGO or Pd-NPs only. The response of the sensor to DA is linear in the 1–150 μM concentration range, and the detection limit is 0.233 μM. The sensor was applied to the determination of DA in commercial DA injection solutions.

Schematic representation showing the oxidation of DA at RGO-Pd-NPs composite electrode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurotransmitters have received considerable attention due to the key roles played by them in the central nervous systems of our body [1]. Dopamine (DA) is one of the major catecholamine that exists in the central nervous system, which regulates our cognition and emotions in our body [2]. Moreover, dopaminergic neurotransmission leads to disorders like Parkinson’s disease. Hitherto, various techniques have been used for sensitive detection of DA that includes high performance liquid chromatography (HPLC) [3], mass spectrometry [4] spectrophotometry [5], and electrochemical methods [6–8]. However, electrochemical methods are very simple, cost-effective, highly sensitive, and user friendly than other traditional methods [9–11]. Moreover, electrochemical methods provide high selectivity towards detection of DA. So far, carbon nanomaterials, metal nanoparticles [12], polymers [13, 14] and metal oxides [15] have been used as electrocatalysts for the detection of DA. Among various carbon nanomaterials, reduced graphene oxide (RGO) has received considerable attention in the field of electroanalytical chemistry and it has been used as electrocatalysts for sensing numerous analytes. The higher surface area and superior conductivity of RGO favored the detection of biological analytes with high specificity. In recent years, RGO-metal nanoparticle (NPs) composites have perceived significant interest, likely because of their excellent sensing performances that open up various potential applications in the field of electrochemical sensors [16]. Pd, Au, Cu and Ag NPs decorated directly onto RGO sheets have been proven to be potential sensing platforms for the detection of various analytes [17]. However, chemical or thermal methods have been employed for the preparation of RGO-metal NP composites, in which both GO and metal precursors were reduced simultaneously using conventional reducing agents [18]. In particular, Pd NPs has been extensively used for the detection of many biological analytes due to its good electrocatalytic ability [19]. Compared with other approaches that used for the preparation of RGO from graphene oxide (GO), electrochemical reduction method is considered to be more simple, green (no toxic byproducts) and cost-effective [20]. Moreover, electrochemical approach allows preparation of RGO-Pd-NPs composite through electro reduction of GO and Pd2+ ions in a single step under cathodic conditions. Many reports on decoration of Pd-NPs directly onto RGO sheets via two steps through chemical or electrochemical method are available in the literature [21]. However, there are only few reports about single step electrochemical preparation of RGO-Pd-NPs composite [22]. Herein, we reported on the synthesis of RGO-Pd-NPs composite via potentiostatic method based on the simultaneous reduction of Pd2+ ions and GO, which is more facile and less time consuming than other existing methods. We investigated the electrochemical determination of DA at electrochemically prepared RGO-Pd-NPs composite modified electrode for the first time. To the best of our knowledge, RGO-Pd-NPs composite has never been used for the electrochemical determination of DA. The high surface area and good conductivity of the RGO-Pd-NPs composite allows efficient oxidation of DA. Moreover, composite electrode showed greater electrocatalytic oxidation for DA than RGO and Pd-NPs modified electrodes. Additionally, the practicality of this sensor towards the detection of DA in commercial DA injection solutions has also been demonstrated.
Experimental
Materials and methods
Raw graphite and PdCl2 were purchased from Aldrich (http://www.sigmaaldrich.com/taiwan.html). Dopamine hydrochloride, dopamine hydrochloride injections were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com/taiwan.html) and used as received. The phosphate buffer solution (PB) at pH 7 was prepared by using 0.05 M Na2HPO4 and NaH2PO4 solutions and pH of the solutions was adjusted with 0.5 M H2SO4 and 2 M NaOH. All other chemicals were used of analytical grade and all solutions were prepared using Millipore water.
All electrochemical measurements were carried out by CHI 750a electrochemical analyzer (CH instruments). Linear sweep voltammetry (LSV) was performed by CHI 250a electrochemical analyzer (CH instruments). Surface morphological studies were carried out using Hitachi S-3000 H scanning electron microscope. Electrochemical impedance spectroscopy (EIS) studies were performed by using IM6ex ZAHNER (Kroanch, Germany). Glassy carbon electrode (GCE) was used as a working electrode. Ag/AgCl electrode (Sat. KCl) and platinum wire was used as reference and counter electrodes, respectively. All measurements were carried out at room temperature and solutions in the electrolyte cell were kept under an inert atmosphere.
Fabrication of RGO-Pd-NPs composite
Graphite oxide was prepared from raw graphite by using Hummers’ method [23] and it was converted to GO upon subjecting its aqueous solution to ultrasonication for 30 min. In a typical procedure to prepare RGO-Pd-NPs composite, 1 mL of homogenous solution of GO (0.5 mg mL−1) was mixed with PdCl2 (0.05 mM) solution. Then the mixture was sonicated about 20 min at room temperature to attach Pd2+ ions onto GO sheets, which was subsequently centrifuged at 2,000 RPM to remove the free Pd2+ ions that exists in the solution. About 8 μl (optimum concentration) of resulting GO/Pd2+ solution was drop casted onto a GCE surface and then allowed to dry in an air oven. The GO/Pd2+ modified GCE was transferred into an electrochemical cell containing pH 3 solution and a constant potential of −1.1 V was applied for 300 s, which leads to the formation of RGO-Pd-NPs composite. RGO modified GCE was prepared through the electrochemical reduction of GO on GCE without PdCl2. Meanwhile, Pd-NPs was electrodeposited onto GCE surface by using the same procedure but without GO. RGO and Pd-NPs modified GCEs were used as controls. The fabricated RGO-Pd-NPs composite modified GCE was used for further experiments and stored at room temperature under dry condition. Also, the composite was stored at 4 °C in dopamine solution (only for stability studies) when not in use.
Results and discussion
Characterization of RGO-Pd-NPs composite
The SEM image of the RGO-Pd-NPs composite shown in Fig. 1b validates that Pd-NPs are well formed and closely anchored on the RGO sheets. Moreover, Pd-NPs with average diameter of 100–200 nm are clearly observed to be anchored on the RGO sheets. While, RGO sheets depicts wrinkled textures with closely associated ultrathin sheets (A). Likewise, only Pd-NPs with sizes of about 200 nm diameter are also observed (inset in Fig. 1a). It has to be noted that, Pd-NPs are distributed evenly onto RGO sheets, which confirmed the formation of RGO-Pd-NPs composite. Moreover, Pd-NPs electrodeposited on the bare electrode are in good agreement with the Pd-NPs deposited onto RGO. However, Pd-NPs are more closely anchored on RGO sheets without any dislocation, which validates that the RGO-Pd-NPs composite had been formed through the simultaneous reduction of Pd2+ and GO.
The formation of RGO-Pd-NPs composite was further confirmed by the elemental analysis. As it can be seen in the EDX spectrum of RGO-Pd-NPs composite shown in Fig. 2b, the oxygen functionalities of GO are reduced up to 32 %, confirming the formation of RGO from GO. In contrast, only GO/Pd2+ contains higher oxygen content than RGO-Pd-NPs, which confirmed the reduction of GO/Pd2+ leading to the formation RGO-Pd-NPs composite (Fig. 2a). EIS analysis was used to evaluate the conductivity of different modified electrodes. Figure 2c shows the typical EIS of bare (a), RGO (b) and RGO-Pd-NPs (c) modified electrodes acquired in 5 mM Fe2+/Fe3+ probe containing pH 7 solution. RGO-Pd-NPs modified electrode (c) has higher electron conductivity than RGO (b) and bare GC (a) modified electrodes, likely due to its smaller Rct value. The smaller surface area and poor electron conductivity of the bare GCE lead to higher Rct value and higher resistance. Meanwhile, electron conductivity of RGO was found to be greatly enhanced in presence than in the absence of Pd-NPs. All these results clearly suggest that superior conductivity of the Pd-NPs and RGO may lead to faster electron transfer at the electrode surface.
Electrochemical oxidation of DA at RGO-Pd-NPs composite modified electrode
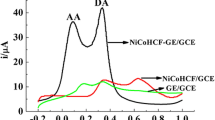
The electrochemical oxidation behavior of DA at different modified electrodes was investigated as shown in Fig. 3. Figure 3 depicts cyclic voltammograms of bare (a), RGO (b), Pd (c) and (d) RGO-Pd-NPs modified GCEs in 100 μM DA in N2 saturated PB at the scan rate of 50 mV s−1. RGO-Pd-NPs modified GCE shows enhanced oxidation peak at 0.234 V for DA than that of RGO modified electrode (0.285 V). The oxidation peak of DA appeared at 0.453 V and 0.266 V for bare and Pd modified electrodes, respectively. Moreover, the oxidation peak current of DA at RGO-Pd-NPs composite was found to be two and three fold higher than that of RGO and Pd modified electrodes. Furthermore, oxidation peak of DA at 0.234 V was found to be much lower than that of other modified electrodes. The superior conductivity and high surface area of the composite modified electrode favored the oxidation of DA at a relatively lower potential. In addition, the presence of Pd-NPs on RGO modified electrode was confirmed by recording cyclic voltammograms at modified electrode in N2 saturated 0.5 M H2SO4 solution at the scan rate of 50 mVs−1. As shown in Fig. 3 (inset), the hydrogen adsorption and desorption peaks of Pd-NPs are clearly seen, whereas oxidation and reduction behavior of Pd was clearly observed at RGO-Pd-NPs composite, which is found to be in good agreement with the electrochemical behavior of Pd-NPs [24].
The effect of scan rate at RGO-Pd-NPs composite modified electrode was studied in the presence of 100 μM DA as shown in Fig. 4. The oxidation peak potential and peak current (Ipa) had linear dependence with scan rates between 20 mV and 100 mV (inset), revealing that the oxidation of DA at RGO-Pd-NPs composite modified electrode is a surface-controlled process, and is not a diffusion controlled process [25]. The pH played a significant role in the oxidation of DA at the composite electrode, so we studied the effect of different pH at RGO-Pd-NPs composite in 100 μM DA in N2 saturated PB at the scan rate of 50 mVs−1 (figure not shown). At the composite electrode both anodic and cathodic peak potentials shifted towards the negative direction upon increasing the pH from 1.0 to 9.0. While they shifted towards positive direction when decreasing the pH 9–1.0, indicating that the electrocatalysis of DA at the composite electrode is a pH dependent reaction. From the calibration curve of anodic peak potential vs different pH, the slope value was calculated to be Epa (V) − 56.23 mV/pH using regression equation. This slope value was found to be in close relationship with the theoretical value of −59 mV/pH at 25 °C for a reversible electron transfer coupled proton transport process that contains equal number of protons and electrons [26]. This result also implied that the oxidation of DA at the composite electrode is an equal number of proton (H+) and electron (e−) process. We also studied the anodic peak current response of DA in different pH solutions. A maximum anodic peak current of DA was observed at pH 7 than in other pH solutions. Therefore, pH 7 was found to be optimal for the oxidation of DA.
Electrochemical determination of DA at RGO-Pd-NPs composite modified electrode
Figure 5 displays the typical LSV curves corresponding to the electrocatalytic oxidation of DA at RGO-Pd-NPs composite modified electrode in N2 saturated PB at the scan rate of 50 mV s−1. As shown in Fig. 5, the oxidation peak current of DA was observed at 0.246 V in the presence of DA. Upon increasing the concentrations of DA in PB, the oxidation peak current increased linearly, but the reduction peak current decreased, revealing the typical electrocatalysis of DA at the composite electrode. The oxidation of DA at the composite modified electrode had linear relationship with DA concentrations ranging from 1 μM to 150 μM with correlation coefficients of 0.997 (inset). The limit of detection was found to be 0.233 μM (S/N = 3) with a sensitivity of 2.62 μA μM−1 cm2. The analytical performances of this dopamine sensor have been compared with other dopamine sensors reported previously as shown in Table 1. It is evident that this sensor showed higher catalytic activity towards DA with a good linear range and low LOD than other graphene and metal NPs based dopamine sensors.
Practicality of the sensor
The practicality of the fabricated sensor was evaluated in commercially available dopamine hydrochloride injection solutions without any further pretreatments. The sensing performance of the sensor was examined by LSV using the same procedure as that mentioned before. The obtained results are shown in the Table 2. It is apparent that the fabricated sensor has great potential to sense DA from both commercial and lab samples, with good recovery results. The results clearly validates that the fabricated sensor could be used to determine DA in the real samples with high precision.
Selectivity and stability of the fabricated electrode
The selectivity of the sensor was investigated by using LSV. The common interfering species like ascorbic acid (AA), glucose and uric acid (UA) were chosen to evaluate the selectivity of the sensor. The interfering species did not affected the dopamine oxidation signals produced at the composite electrode, revealing that the fabricated electrode is highly selective for the determination of DA even when the suspicious species (AA, UA and glucose) existed at higher concentrations of 2 mM. The selectivity of the fabricated electrode is highly useful for the detection of DA in real samples.
We also evaluated the stability, reproducibility and repeatability of the fabricated sensor. For the storage stability study, the sensor was stored in 100 μM DA solution at 4 °C and its oxidation current response was monitored periodically. The sensor retained about 84.9 % of its initial current response even after stored for 2 weeks, revealing the good stability of the sensor. The relative standard deviation (RSD) for the detection of DA at five electrodes was found to be 3.73 %, revealing its good reproducibility. An RSD value of 4.22 % was observed for 10 successive 100 μM DA measurements, showing its good repeatability.
Conclusions
We have fabricated a simple and sensitive dopamine electrochemical sensor based on electrochemically prepared RGO-Pd-NPs composite. The surface morphological studies revealed that Pd nanoparticles with even sizes were formed onto the RGO sheets that had wringed textures. Elemental analysis confirmed that the effective reduction of GO occurred during the electrochemical reduction, leading to the formation of RGO-Pd-NPs composite. The composite electrode showed enhanced electro oxidation behavior for DA than that of other modified electrodes (RGO, Pd and bare modified GCEs). The high surface area and high conductivity of RGO-Pd-NPs composite enhanced the oxidation of DA at the electrode surface. Moreover, the fabricated sensor showed a good response over DA concentrations in the linear range of 1–150 μM with a detection limit of 0.233 μM. In addition, the good recovery results obtained in the commercial DA injections revealed the practicality of the fabricated sensor.
References
Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60:769–779
Heien M, Khan A, Ariansen J, Cheer J, Phillips P, Wassum K, Wightman M (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A 102:10023–10028
Rao PS, Rujikarn N, Luber JM, Tyras DH (1989) A specific sensitive HPLC method for determination of plasma dopamine. Chromatographia 28:307–310
Peaston RT, Weinkove C (2004) Measurement of catecholamines and their metabolites. Ann Clin Biochem 41:17–38
Nezhad MRH, Tashkhourian J, Khodaveisi J (2010) Sensitive spectrophotometric detection of dopamine. Levodopa and adrenaline using surface plasmon resonance band of silver nanoparticles. J Iran Chem Soc 7:83–91
Lai GS, Zhang HL, Han DY (2008) Electrocatalytic oxidation and voltammetric determination of dopamine at a Nafion/carbon-coated iron nanoparticles-chitosan composite film modified electrode. Microchim Acta 160:233–239
Chang HY, Kim DI, Park YC (2006) Electrochemically degraded dopamine film for the determination of dopamine. Electroanalysis 18:1578–1583
Li SJ, Deng DH, Shi Q, Liu SR (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177:325–331
Chang JL, Wei GT, Zen JM (2011) Screen-printed ionic liquid/ preanodized carbon electrode: effective detection of dopamine in the presence of high concentration of ascorbic acid. Electrochem Commun 13:174
Snowden ME, Unwin PR, Macpherson JV (2011) Single walled carbon nanotube channel flow electrode: hydrodynamic voltammetry at the nanomolar level. Electrochem Commun 13:186
Wang Y, Li YM, Tang LH, Lu J, Li JH (2009) Application of graphene-modified electrode for selective detection of dopamine. Electrochem Commun 11:889
Thiagarajan S, Yang RF, Chen SM (2009) Palladium nanoparticles modified electrode for the selective detection of catecholamine neurotransmitters in presence of ascorbic acid. Bioelectrochemistry 75:163–169
Ciszewski A, Milczarek G (1999) Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic acid. Anal Chem 71:1055
Jin GY, Zhang YZ, Cheng WX (2005) Poly(p-aminobenzene sulfonic acid)-modified glassy carbon electrode for simultaneous detection of dopamine and ascorbic acid. Sensors Actuators B 107:528
Zhang WE, Xu B, Jiang LC (2010) Functional hybrid materials based on carbon nanotubes and metal oxides. J Mater Chem 20:6383–6391
Hou S, Kasner ML, Su S, Patel K, Cuellari R (2010) Highly sensitive and selective dopamine biosensor fabricated with silanized graphene. J Phys Chem C 114:14915–14921
Liu C, Wang K, Luo S, Tang Y, Chen L (2011) Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 7:1203–1206
Liu R, Zhou H, Liu J, Yao Y, Huang Z, Fu C, Kuang Y (2013) Preparation of Pd/MnO2-reduced graphene oxide nanocomposite for methanol electro-oxidation in alkaline media. Electrochem Commun 26:63–66
Chen ZH, Jie JS, Luo LB, Wang H, Lee CS, Lee ST (2007) Applications of silicon nanowires functionalized with palladium nanoparticles in hydrogen sensors. Nanotechnology 18:345502–345507
Palanisamy S, Chen SM, Sarawathi R (2012) A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sensors Actuators B 166–167:372–377
Lu LM, Li HB, Qu F, Zhang XB, Shen GL, Yu RQ (2011) In situ synthesis of palladium nanoparticle–graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens Bioelectron 26:3500–3504
Nagaraju DH, Suresh GS (2012) Green chemistry route to the synthesis of palladium nanoparticles on reduced graphene oxide for ethanol fuel cells applications. ECS Electrochem Lett 1:21–23
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Jiang Y, Lu Y, Li F, Wu T, Niu L, Chen W (2012) Facile electrochemical codeposition of “clean” graphene–Pd nanocomposite as an anode catalyst for formic acid electrooxidation. Electrochem Commun 19:21–24
Lai GS, Zhang HL, Jin GM (2007) Electrocatalysis and voltammetric determination of dopamine at a calix[4]arene crown-4 ether modified glassy carbon electrode. Electroanalysis 19:496–501
Aoki K, Tokuda K, Matsuda H (1987) Theory of stationary current-potential curves at micro disk electrodes for quasi-reversible and totally irreversible electrode reactions. J Electroanal Chem 235:87–96
Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, Kim JS, Kim H (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25:2366–2369
Wang Y, Peng W, Liu L, Tang M, Gao F, Li M (2011) Enhanced conductivity of a glassy carbon electrode modified with a graphene-doped film of layered double hydroxides for selectively sensing of dopamine. Microchim Acta 174:41–46
Zhang F, Li Y, Gu Y, Wang Z, Wang C (2011) One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173:103–109
Wang AJ, Feng J, Li YF, Xi JL, Dong WJ (2010) In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis toward dopamine. Microchim Acta 171:431–436
Acknowledgments
This project was supported by the National Science Council and the Ministry of Education of Taiwan (Republic of China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palanisamy, S., Ku, S. & Chen, SM. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim Acta 180, 1037–1042 (2013). https://doi.org/10.1007/s00604-013-1028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1028-1