Abstract
We report on a method for the extraction of the lanthanide ions La(III), Sm(III), Nd(III) and Pr(III) using a carbon-ferrite magnetic nanocomposite as a new adsorbent, and their determination via flow injection ICP-OES. The lanthanide ions were converted into their complexes with 4-(2-pyridylazo)resorcinol, and these were adsorbed onto the nanocomposite. Fractional factorial design and central composite design were applied to optimize the extraction efficiencies to result in preconcentration factors in the range of 141–246. Linear calibration plots were obtained, the limits of detection (at S/N = 3) are between 0.5 and 10 μg L−1, and the intra-day precisions (n = 3) range from 3.1 to 12.8 %. The method was successfully applied to a certified reference material.

Superparamagnetic activated carbon based nanocomposite was synthesized and applied for extraction and determination of some rare earth elements in water samples
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lanthanides are widely distributed in low concentrations throughout the earth’s crust and are known as the part of rare earth elements (REE). In modern societies, REEs due to their metallurgical, optical and electronic properties are widely used in several industries such as ceramics, semiconductors, magnets, polishing compounds, carbon arcs and so forth [1]. Therefore, the development of analytical methodologies for the separation and determination of these elements becomes essential. The several analytical techniques were applied to determine lanthanides in samples such as neutron activation (INAA) [2], ion chromatography [3], inductively coupled plasma-mass spectrometry (ICP-MS) [1], energy dispersive X-ray fluorescence (EDXRF) [4] and inductively coupled plasma optical emission spectroscopy (ICP-OES) [5]. By taking advantage of multi-elemental detection capabilities along with high sensitivity of ICP-OES instrument, this method can be successfully applied for the determination of REE in some environmental samples [6]. But low levels of REE concentration in real samples are not compatible with the detection limit of this technique.
The most widely used techniques for the separation and preconcentration of trace elements include various types of liquid-liquid extraction, solid-phase extraction, coprecipitation, floatation and etc. Recently, the solid-phase extraction (SPE) technique has become increasingly popular in trace elements preconcentration compared with the classical liquid-liquid extraction method because of its advantages of high enrichment factor, high recovery, rapid phase separation, low cost, low consumption of organic solvents and the ability to combine with different detection techniques in on-line or off-line mode [7].
Numerous substances have been proposed and applied as solid-phase extraction sorbents for preconcentration of REEs from dilute solutions, such as multiwalled carbon nanotube [8], Muromac A-l [9], iminodiacetate-based resin [10], 8-quinolinol-immobilized fluorinated metal alkoxide glass [MAF-8HQ] [11], Chelex 100, activated alumina, activated carbon, HDEHP/H2MEHP (Di-2-ethylhexyl hydrogen phosphate/2-ethylhexyl dihydrogen phosphate) adsorbed on C18 cartridge [12], microcrystalline naphthalene [13] and octadecylsilica [14].
Magnetic nanoparticles are a class of nanoparticles which can be manipulated using magnetic field. Thanks to the easy separation by applying a magnetic field, magnetic nanoparticles have a good potential for treatment of contaminated water. Recently, Yang et al. have developed magnetic Fe3O4-activated carbon nanocomposite particles for removal of methylene blue from aqueous solution [15].
In this work, activated carbon based magnetic nanocomposite (AC-MNC) with exploitable characteristics was synthesized and used as adsorbent for preconcentration of lanthanum, samarium, neodymium and praseodymium ions from aqueous solution. These magnetic nanocomposite adsorbent consist of commercially available activated carbon and magnetic iron oxide nanoparticles. Furthermore, they were carefully characterized before investigating their adsorption properties for the separation and preconcentration of these elements.
4-(2-Pyridylazo)-resorcinol (PAR) is a heterocyclic azo compound that has been used as a chelating agent for the SPE separation and preconcentration of lanthanide ions. Lanthanide ions form stable complexes with PAR and in the extraction procedure the formed complexes are adsorbed on AC-MNC [16, 17]. The sorbent were then collected using an external magnetic field. Afterward, the extracted metals ions were washed from the surface of the adsorbent and determined simultaneously using a flow injection ICP-OES. To the best of our knowledge, AC-MNC has not been employed previously for the extraction and determination of the studied lanthanide ions from aqueous samples.
Experimental
Standard solutions and reagents
The lanthanides stock solutions (1,000 mg L−1) were prepared by dissolving their oxides in 0.1 mol L−1 of nitric acid and diluted with 2 % (v/v) HNO3. Standard solutions and test solutions were obtained by diluting the stock solutions. The ligand solution (0.01 mol L−1) was prepared by dissolving analytical reagent grade PAR in high purity water. All chemicals were analytical grade and were obtained from Merck (Darmstadt, Germany; www.merck-chemicals.com). The pH of solutions was adjusted by drop wise addition of NH3 (0.1 mol L−1) and/or HCl (0.1 mol L−1) solutions. High purity water was used throughout of the study.
Instrumentation
The measurements were performed with a Perkin Elmer Optima 2100 DV simultaneous inductively coupled plasma optical emission spectrometry (ICP-OES) (Shelton, USA; www.perkinelmer.com) coupled to a pneumatic nebulizer and equipped with a charge coupled device (CCD). Operational conditions and selected wavelengths for the lanthanide ions were optimized and summarized in Table 1. A sample loop (60 mm length × 4 mm i.d.) was prepared from silicon tube and was used for flow injection introduction of the preconcentrated phase into the nebulizer of ICP-OES. A six-way two position injection valve (homemade) (Fig. S1, Electronic Supplementary Material) was used in the injection/elution process. The pH of solutions was measured by a Corning ion analyzer 255 pH meter (New York, USA; www.corning.com) with combined glass electrode supplied with a combined glass-calomel electrode. Transmission electron microscopy (TEM) images of the magnetic nanocomposite were obtained with a Philips CM120 transmission electron microscope (Eindhoven, Netherlands; www.fei.com) and thermogravimetric analysis (TGA) of the adsorbent was performed by FTDA/TGA instrument from Mettler Toledo (Boston, England; www.mt.com/lab). The magnetic properties of the nanocomposite were measured using a homemade vibrating sample magnetometer (University of Esfahan, Iran). The high purity water was prepared on a Millipore water purification system (Billerica, MA, USA; www.millipore.com).
Preparation of certified soil sample
The sample preparation procedure for the leaching of trace metals from the certified reference material (CRM) was based on the method that was described by Kadi [18]. An accurate weight (0.48 ± 0.01 g) of CRM (IAEA Soil-7) sample was transferred into a Teflon beaker (100 mL) containing HF (28.0 mL), concentrated HCl (8.0 mL), and concentrated HNO3 (15.0 mL) at room temperature to digest the sample gradually and slowly. The reaction mixture was then heated slowly for 1 h at 100–150 °C on a hot plate. After the evolution of NO2 fumes had ceased, the reaction mixture was evaporated almost to dryness and mixed again with concentrated HNO3 (20.0 mL). The process was repeated thrice and the mixture was again evaporated to dryness. After evaporation, the solid residue was then re-dissolved in dilute nitric acid (10.0 mL, 1.0 mol L−1). The resulting mixture was filtered through a Whatman filter paper, transferred to volumetric flask (100 mL) and the solution was diluted to the mark with high purity water.
Preparation and characterization of AC-MNC
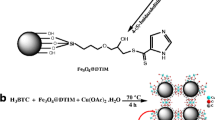
The magnetic nanocomposite was prepared based on the method that was described by Yang and Zho [15]. At the first step activated carbon (AC) was modified with nitric acid (65 %) for 3 h at 80 °C to make it hydrophilic. Then, 1 g of the modified AC was dispersed in 40 mL aqueous solution containing 8 g Fe(NO3)3·9H2O and stirred for 45 min. The resulted mixture filtrated and dried. Afterwards modified activated carbon that adsorbed iron salt, was heated at 750 °C for 3 h in the presence of argon for the formation of magnetic nanoparticles of Fe3O4 that are obtained by combustion synthesis in the pores of activated carbon [19]. The obtained activated carbon based on magnetic nanocomposite (AC-MNC) was stable under environmental conditions for several months. TEM image of the prepared AC-MNC that is shown in Fig. 1a demonstrates the existence of iron oxide nanoparticles with diameter of about 40–80 nm. The magnetic hysteresis loop of AC-MNC that was measured by vibrating sample magnetometer (VSM) is shown in Fig. 1b. From the plotting of magnetization (M) vs magnetic field (H), very weak hysteresis revealed the resultant magnetic nanoparticles were nearly superparamagnetic with a saturation magnetization (Ms) of 7.28 emu g−1 at room temperature. Thermogravimetric analysis (TGA) of the magnetic nanoparticles (Fig. 1c) indicated that there was about 9 wt % of iron oxide inside the synthesized nanocomposite.
SPE procedure
An aliquot of 100 mL of water sample (pH 8.0) was transferred to 100 mL glass beaker. Then, 0.4 mL of 0.01 mol L−1 PAR and 25 mg of sorbent were added into it and the resulted mixture was shaken completely. The mixtures were stirred and allowed to complete the extraction process for 15 min. Subsequently, an Nd-Fe-B strong magnet (10 cm × 5 cm × 4 cm, 1.47 T) was placed at the bottom of the beaker, and the magnetic nanocomposite was collected from the solutions. After about 3 min, the solutions became clear and supernatant solutions were decanted. Finally, La3+, Sm3+, Nd3+ and Pr3+ complexes were eluted from the sorbent using 0.4 mL of acidic propylene carbonate (PC) and 0.2 mL of this solution was injected into the ICP-OES using the six-way two-position injection valve equipped with a silicon injection loop (V = 500 μL). The high purity water was used as carrier to transfer the acidic propylene carbonate into the ICP-OES.
Experimental design
The statistical computer package “Statgraphics Plus 5.1” (Manugistics Inc, MA, USA; www.statgraphics.com) was used. The software statistically analyses the experimental data (e.g. ANOVA, determination of the estimated effects and interaction, regression equation which has been fitted to the data) and plots the Pareto chart and the estimated response surface. Two types of experimental design have been used for this study:
-
1.
A screening design followed by fractional factorial design (FFD) to define the most effective parameters.
-
2.
A response surface design for optimization of the effective parameters.
FFD was employed as a screening design. In this way, the number of experiments was kept low based on the assumption that interaction effects between three or more parameters are small compared to main and two-variable interaction effects. Thus, it is possible to select a fraction of the full factorial design and omit several combinations of parameters from experimental plan [20]. In our study the FFD involved 32 experiments, carried out randomly in order to estimate the experimental error and the significance of each variable.
Afterwards, from the results of the FFD, a central composite design (CCD) was built using the same variables as in the FFD except those were ineffective parameters. The total number N of experiments with f factors is: N = 2f + 2f + N0. The first term is related to the full factorial design, the second to the star points and the third to the center point. The length of the arms of the star (α) played a major role for the appearance of the CCD. Based on α value, there are two different designs for CCD: Face center central composite design (FCCCD) whit ∣α∣ = 1 and circumscribed central composite design (CCCD) whit ∣α∣ > 1 that investigated the variables at three and five levels, respectively. All other experiments were performed randomly and without replication. Finally, the response surface plots were presented in order to visualization and rapid selection of optimal conditions. Response surface plots are presented in three-dimensional space and clearly show the influence of two factors on the extraction efficiency in the investigated as well as slightly outside of the investigated region [21].
Results and discussion
Optimization of the SPE variables
Different parameters such as type of eluent, volume and pH of sample solution, stirring rate, extraction time, ligand amount and eluent volume can affect the lanthanides extraction efficiency. In the first stage of the study, several experiments were conducted in order to decide which variables should be included in the screening design. Investigation of type of eluent in screening design experiments is time consuming and increases the number of runs. Study the effect of this parameter separately can reduce the number of runs. Therefore, this parameter was separately investigated at the first stage.
Type of eluent
The adsorbed complexes of metal ions on the surface of sorbent can be quantitatively eluted using organic solvents [22, 23]. Methanol, ethanol, propanol, acidic propanol, acetonitrile and 9 % (v/v) acetic acid in propylene carbonate (PC) were tested as the eluents. Among the tested organic solvents, 400 μL of acidified PC was found to be compatible with ICP-OES and the superior solvent in comparison with other solvents for desorption of complexes of the metal ions from the surface of AC-MNC. So acidified PC was chosen as the best eluent for the desorption of of lanthanides complexes from AC-MNC in subsequent experiments.
Selection of most effective parameters
The FFD (32 experiments) was used to estimate the most effective parameters on the extraction efficiency. The analysis of the results based on the total normalized ICP-OES intensity of the metal ions is visualized using a standardized main and two factor interactions effect Pareto charts (P < 0.05) [24]. As shown (Fig. S2), the most important factors affecting the extraction effiency are pH of sample solution (A), extraction time and PAR amount simultaneously (BD). Thus three factors (A, B and D) were considered as effective parameters. The other variables (volume of sample solution, stirring rate and eluent volume) and all the other interactions are not significant factors in the studied range.
Optimization of effective parameters using CCCD
In order to find the best extraction conditions, experimental designs were performed based on a CCCD to optimize conditions and for each variable assumed five levels (−α, −1, 0, +1, +α) [25] to obtain the response surfaces for the aforementioned significant variables and to calculate the optimal values. The investigated variables, their domains and obtained optimal values are presented in Table 2.
The summary of the analysis of variance (ANOVA) is shown in Table S1 (Electronic Supplementary Material). The ANOVA of regression model demonstrates that the model is significant. The goodness of the model can be checked by the coefficient of determination (R2). Based on Joglekar and May [26], least value of R2 must be 0.8 (for acceptable accordance of the model). The value of R2 (0.83) indicates good relation between the experimental and predicted values of the response.
The Eq. 1 illustrates the relation of the three variables (i.e. pH, extraction time (t) and PAR amount (R)) and normalized intensity.
For better visualization and rapid choose of true optimum conditions surface plots were constructed. The representative plots for both substances are presented in Fig. 2 in which optimal conditions, the interactions between variables and their mutual dependence can be clearly noticed. Figure 2 shows the response surface of the pH and the reagent amount (Fig. 2a), the reagent amount and the time (Fig. 2b) and the pH and the time (Fig. 2c). In the graphs it is clearly seen, that pH = 8, contact time of 15 min and PAR to the each metal ion ratio of 4 gives the highest extraction efficiency. So optimum values of effective parameters are determined by the software which are in agreement with response surface plots.
The pH not only plays an important role in the complexation of the metal ions with PAR but also affects the surface charge of the adsorbent. Due to existence of carboxylic group on the surface of AC-MNC [19], the surface charge of AC-MNC became negative by increasing pH of the solution to pH > 7 and an electrostatic attraction occurred between the positively charged metal ions complexes and the adsorbent particles. Also, optimum pH for complexation of the lanthanide ions with PAR is between 6.0 and 8.0 [27, 28]. Formation of cationic PAR complexes enhances adsorption of the metal ions on the negative surface of the adsorbent at alkaline conditions. At pH higher than 8.0, the extraction efficiency was constant, thus pH = 8.0 was chosen for further studies. PAR can form very stable complexes with lanthanide ions and reaction between lanthanide ions and PAR is so fast [27].
As it can be seen in Fig. 2, the recovery increases up to an L/M = 4 and reaches near a quantitative extraction at this molar ratio. At higher L/M ratios (>4), the extraction recovery decreased, perhaps due to the competition of PAR and metal complex for their adsorption on AC-MNC [22]. So an L/M = 4 was chosen for subsequent experiments.
Optimal extraction time is necessary to complete extraction. Results showed that 15 min is sufficient time for maximum quantitative extraction.
Interference studies
The effect of the interfering ions on the extraction efficiency of REEs was studied using solution containing 100 μg L−1 of REEs prepared in the presence and absence of interference species. Table 3 shows the tolerance limits of the interfering ions. Results showed that Zn2+, Ni2+ and PO4 3− can interfere in the extraction and determination of lanthanide ions at a higher concentration levels. However, by using higher amounts of PAR (L/M > 4) and adsorbent, the interference effect of them on the extraction of lanthanide ions was decreased. The concentrations of the other studied metal ions in real samples (CRM) are lower than the tolerance limits reported for them.
Performance of the presented method
The analytical characteristics of the presented method (i. e. enhancement factors (EFs), limit of detections (LODs), linear dynamic ranges (LDRs) and precision (RSDs)) were obtained under optimized conditions and were tabulated in Table 4. To evaluate the linearity of the method, 11 spiking level of La3+, Sm3+, Nd3+ and Pr3+ ions in the concentration range of 2–600 μg L−1 were used and LDRs in the range of 2–500 μg L−1 were obtained. LOD for each lanthanide ion was obtained from CDL = kSb/m where k = 3, Sb is the standard deviation of three replicate blank measurements and m is the slope of calibration curve. The EFs which were calculated as the ratio of the slopes of the preconcentration and direct calibration equations varied between 141 and 246. Some characteristics of previously reported methods such as extraction time, LODs, LDRs and EFs for extraction and determination of lanthanides are summarized in Table 5 for comparison. LODs and RSDs % were between 0.5–10 μg L−1 and 3.1–12.8 %, respectively. As can be seen, LODs, LDRs, RSDs % and Efs of the current method are appropriate and comparable with the other methods that were reported for preconcentration and determination of lanthanides.
Method validation and analysis of soil sample
The presented method was validated by analyzing CRM sample (IAEA-soil-7) after digestion of it based on the method that was mentioned in the experimental section. This method was applied for the determination of La3+ ion that was presented in CRM sample. For obtaining the results with maximum accuracy, the standard addition method was applied by extracting from 100 mL of the spiked solutions (pH = 8; Vs = 0, 0.5, 1, 1.5 mL and Cs = 10 mg L−1). Concentration of inorganic lanthanum in IAEA-soil-7 was found to be 30.8 ± 0.5 μg g−1 which is in agreement with the certified value of 28 ± 0.4 μg g−1 for total lanthanum.
Conclusion
In the present work, a simple, sensitive and reliable SPE-FI-ICP-OES method was developed using activated carbon based magnetic nanocomposite as a new solid phase extraction sorbent for preconcentration of La3+, Sm3+, Nd3+ and Pr3+ ions in aqueous solutions. Compared with the commonly used sorbents, this sorbent can be easily prepared and also has super paramagnetic property and can be collected after extraction by using a magnet. In addition, the retained complexes of lanthanide ions on AC-MNC can be easily desorbed by acidified propylene carbonate. A low limit of detection, up to 0.5 μg L−1 and enhancement factors of 141–246 are the main advantages of this analytical procedure in comparison with similar ICP-OES methods. The results demonstrated that the method is very suitable for the rapid extraction of lanthanide ions from aqueous solutions at ppb levels besides having both good accuracy and precision. The method was validated by determination of the analytes concentration in the certified soil sample and satisfactory results were obtained. The obtained results showed that it is possible to obtain better LODs by extraction of the analytes from large volumes of the sample solution and using the flow injection method to introduce smaller volumes of the eluted solution of the analytes into the ICP nebulizer. Accordingly, the presented method can be a guideline for the extraction and determination of trace amounts of REEs in biological and environmental samples. The procedure is suitable for extraction of REEs from complex matrices like as soil samples.
References
Raut NM, Huang LS, Aggarwal SK, Lin KC (2003) Determination of lanthanides in rock samples by inductively coupled plasma mass spectrometry using thorium as oxide and hydroxide correction standard. Spectrochim Acta Part B 58:809–822. doi:10.1016/S0584-8547(03)00016-8
Wang YQ, Sun JX, Chen HM, Guo FQ (1999) Determination of the contents and distribution characteristics of REE in natural plants by NAA. J Radioanal Nucl Chem 219:99–103. doi:10.1007/BF02040273
Dybczynski SR, Czerska E, Danko B, Kulisa K, Samczynski Z (2010) Comparison of performance of INAA, RNAA and ion chromatography for the determination of individual lanthanides. Appl Radiat Isot 68:23–27. doi:10.1016/j.apradiso.2009.09.002
Cornejo-Ponce L, Peralta-Zamora P, Bueno MS (1998) Preconcentration of rare earths using silica gel loaded with 1-(2-pyridylazo)-2-naphthol (PAN) and determination by energy dispersive X-ray fluorescence. Talanta 46:1371–1378. doi:10.1016/S0039-9140(98)00002-2
Premadas A, Srivastava PK (2001) Inductively coupled plasma atomic emission spectrometric determination of lanthanides and Y in various uranium hydrometallurgical products. J Radioanal Nucl Chem 251:233–239. doi:10.1023/A:1014804007992
Shariati S, Yamini Y, Faraji M, Saleh A (2009) On-line solid phase extraction coupled to ICP-OES for simultaneous preconcentration and determination of some transition elements. Microchim Acta 165:65–72. doi:10.1007/s00604-008-0099-x
Pyrzynska K, Trojanowicz M (1999) Functionalized cellulose sorbents for on-line preconcentration of trace metals for environmental analysis. Crit Rev Anal Chem 29:313–332. doi:10.1080/10408349891199329
Liang P, Liu Y, Guo L (2005) Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim Acta B60:125–129. doi:10.1016/j.sab.2004.11.010
Hirata S, Kajiya T, Aihara M, Honda K, Shikino D (2002) Determination of rare earth elements in seawater by on-line column preconcentration inductively coupled plasma mass spectrometry. Talanta 58:1185–1194. doi:10.1016/S0039-9140(02)00418-6
Willie SN, Sturgeon RE (2001) Determination of transition and rare earth elements in seawater by flow injection inductively coupled plasma time-of-flight mass spectrometry. Spectrochim Acta B 56:1707–1716. doi:10.1016/S0584-8547(01)00263-4
Kajiya T, Aihara M, Hirata S (2004) Determination of rare earth elements in seawater by inductively coupled plasma mass spectrometry with on-line column pre-concentration using 8-quinolinole-immobilized fluorinated metal alkoxide glass. Spectrochim Acta B 59:543–550. doi:10.1016/j.sab.2003.12.01
Bahramifar N, Yamini Y (2005) On-line preconcentration of some rare earth elements in water samples using C18-cartridge modified with l-(2-pyridylazo) 2-naphtol (PAN) prior to simultaneous determination by inductively coupled plasma optical emission spectrometry (ICP-OES). Anal Chim Acta 540:325–332. doi:10.1016/j.aca.2005.03.058
Cai B, Hu B, Xiong H, Liao Z, Mao L, Jiang Z (2001) Preconcentration of lanthanum, europium and ytterbium with tribromoarsenazo-cetylpyridinium bromide supported on microcrystalline naphthalene and their determinations by inductively coupled plasma atomic emission spectrometry. Talanta 55:85–91. doi:10.1016/S0039-9140(01)00394-0
Karami H, Mousavi MF, Yamini Y, Shamsipur M (2004) On-line preconcentration and simultaneous determination of heavy metal ions by inductively coupled plasma-atomic emission spectrometry. Anal Chim Acta 509:89–94. doi:10.1016/j.aca.2003.12.022
Yang N, Zhu S, Zhang D, Xu S (2008) Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater Lett 62:645–647. doi:10.1016/j.matlet.2007.06.049
Sommer L, Novotná H (1967) Complexation of aluminium, yttrium, lanthanum and lanthanides with 4-(2-pyridylazo) resorcinol (par). Talanta 14:457–471. doi:10.1016/0039-9140(67)80072-9
Ohyoshi E (1984) Spectrophotometric determination of formation constants of 1:1 complexes of lanthanides with 4-(2-pyridylazo) resorcinol (par). Talanta 31:1129–1132. doi:10.1016/0039-9140(84)80264-
Kadi MW (2009) Soil pollution hazardous to environment: a case study on the chemical composition and correlation to automobile traffic of the roadside soil of Jeddah city. J Hazard Mater 168:1280–1283. doi:10.1016/j.jhazmat.2009.03.015
Toniolo J, Takimi AS, Andrade MJ, Bonadiman R, Bergmann CP (2007) Synthesis by the solution combustion process and magnetic properties of iron oxide (Fe3O4 and a-Fe2O3) particles. J Mater Sci 42:4785–4791. doi:10.1007/s10853-006-0763-7
Preu M, Guyot D, Petza M (1998) Development of a gas chromatography-mass spectrometry method for the analysis of aminoglycoside antibiotics using experimental design for the optimization of the derivatisation reactions. J Chromatogr A 818:95–108. doi:10.1016/S0021-9673(98)00537-8
Zivanović L, Canski AL, Cević MZ, Jocić B, Kostić M (2008) Application of experimental design in optimization of solid phase extraction of mycophenolic acid and mycophenolic acid glucuronide from human urine and plasma and SPE-RP-HPLC method validation. J Pharm Biomed Anal 47:575–585. doi:10.1016/j.jpba.2008.01.046
Faraji M, Yamini Y, Rezaee M (2010) Extraction of trace amounts of mercury with sodium dodecyle sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 81:831–836. doi:10.1016/j.talanta.2010.01.023
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–177. doi:10.1016/j.aca.2009.11.053
Martendal E, Budziak D, Carasek E (2007) Application of fractional factorial experimental and Box-Behnken designs for optimization of single drop microextraction of 2,4,6-trichloroanisole and 2,4,6-tribromoanisole from wine samples. J Chromatogr A 1148:131–136. doi:10.1016/j.chroma.2007.02.079
Nowak M, Seubert A (1999) Application of experimental design for the characterization of a novel elution system for high capacity anion-chromatography with suppressed conductivity detection. J Chromatogr A 855:91–109. doi:10.1016/S0021-9673(99)00683-4
Korany MA, Fahmy OT, Mahgoub H, Maher HM (2011) High performance liquid chromatographic determination of some guaiphenesin-containing cough-cold preparations. J Adv Res 2:121–130. doi:10.1016/j.jare.2010.09.005
Safronikhin A, Ehrlich H, Shcherba T, Kuzmina N, Lisichkin G (2011) Formation of complexes on the surface of nano sized europium fluoride. Colloid Surf A Physicochem Eng Aspects 377:367–373. doi:10.1016/j.colsurfa.2011.01.034
Shi Y, Eyring EM, Eldik RV (1998) Kinetics and mechanisms of the complexation of aqueous lanthanide ions by 4-(2-pyridylazo) resorcinol. J Chem Soc Dalton Trans 21:3565–3576. doi:10.1039/A805808C
Itoh J, Liu J, Komata M (2006) Novel analytical applications of porphyrin to HPLC post-column flow injection system for determination of the lanthanides. Talanta 69:61–67. doi:10.1016/j.talanta.2005.08.056
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 230 kb)
Rights and permissions
About this article
Cite this article
Tajabadi, F., Yamini, Y. & Sovizi, M.R. Carbon-based magnetic nanocomposites in solid phase dispersion for the preconcentration some of lanthanides, followed by their quantitation via ICP-OES. Microchim Acta 180, 65–73 (2013). https://doi.org/10.1007/s00604-012-0913-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0913-3