Abstract
We have prepared an environmental friendly sorbent by modifying multi-walled carbon nanotubes with tannic acid. The adsorption of La (III), Tb (III) and Lu (III) as a function of contact time, initial solution pH, and quantity of adsorbent was studied using a batch technique. Both Langmuir and Freundlich isotherms can be used to describe the process. The major adsorption mechanisms were attributed to ion exchange and surface complexation. The kinetics of the adsorption follows a pseudo-second-order model. The thermodynamic functions ΔH, ΔG, and ΔS indicate that the sorption is endothermically driven. The adsorbed ions can be readily desorbed from the surface with 1 M hydrochloric acid.

An environmental friendly sorbent, multi-walled carbon nanotubes modified with tannic acid has been prepared and used for the adsorption of REEs. Adsorption capacity, Langmuir and Freundlich adsorption isotherms, kinetics, and thermodynamic functions have been investigated. The major adsorption mechanism can be attributed to ion exchange and surface complexation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earths elements (REEs) have obtained considerable attention because of their unique properties, such as the production of superconductors, super-magnets and geochemical natures. REEs are important elements not only in industrial applications but also in energy and environmental problems [1]. It is essential to find appropriate methods to satisfy the needs for quality control, certification, and evaluation of material performance.
The most widely used techniques for the separation and removal of trace REEs include precipitation [2], solvent extraction [3], adsorption [4] and ion-exchange [5], etc. Although solvent extraction has been widely used since effective extraction ability and separation selectivity, the large amount of organic solution strongly destroys the environment and harms human health.
Among the various methods, adsorption technique is undoubtedly the most frequently used method. As an economical and efficient method, adsorption technique has found its extensive applications to this field, in which many kinds of sorbents are used as adsorption materials including inorganic oxides [6–9], zeolites [10], silica [11–15], activated carbon [16], various resins, [4, 5, 17–24], biological adsorbents [25–27], and etc. [28, 29]. In our previous work [5], a sensitive and rapid on-line method has been developed for the determination of trace amounts REEs by microwave plasma torch-atomic emission spectrometry (MPT-AES) combined with micro-column filled with a strong basic cinnamene anion exchange resins for matrix elimination and enrichment of the analytes. The adsorbed metal ions are eluted and transferred into the detector with nitric acid solutions for the simultaneous multi-element determination. The method has been validated through measurements of La, Ce, Nd, and Y in high purity REE oxide samples.
In recent years, great attention has been paid to the application of nano-structure materials, especially carbon nanotubes (CNTs). Due to their high mechanical strength, large specific surface area, high chemical and thermal stabilities, and low cost, CNTs have been widely used as a promising adsorbent for the removal of metal ions or organic contaminants [30–32]. In addition, their surface properties can be modified through chemical treatments to satisfy some special needs [33–35]. Based on the structure of CNTs, they can introduce oxygen-containing negatively functional groups such as –COOH, –OH, or –C = O on their surface site after treatment with oxidants. In order to obtain high adsorption capacity, it is being paid more and more attention to find an effective modification method of CNTs. Tannic acid (TA, C76H52O46), with several o-dihydroxy and trihydroxy aromatic rings (polyhydroxy polyphenols), has attracted much attention because of their metal-binding properties, antimicrobial and anticorrosive activities in aqueous solutions [36, 37]. Furthermore, some previous studies have indicated that TA may be adsorbed onto CNTs surface with a sorption affinity comparable to that of dissolved organic matter [33–35]. All the facts mentioned above reveal to us that CNTs modified with TA may have great potential as an effective adsorbent. However, such study has received little attention so far.
The basic objectives of the present work are: (1) to study the adsorption of TA with multi-walled carbon nanotubes (MWCNTs) for optimizing the modification method of MWCNTs with TA; (2) to investigate the adsorption of REEs with the synthesized TA-MWCNTs by varying experimental parameters, such as pH, initial concentration, contact time, and temperature, etc.; (3) to obtain the adsorption isotherms and kinetics of REEs with TA-MWCNTs.
Experimental section
Reagents and materials
MWCNTs were purchased from Chengdu Organic Chemicals Co., Ltd., Chinese Academy of Sciences, (http://www.cioc.ac.cn/). The main range of the outer diameter, length and special surface area of MWCNTs are 20 to 30 nm, 30 to 40 μm, and 110 to 300 m2 g–1, respectively. High purity La2O3 (99.999%), Tb2O3 (99.999%) and Lu2O3 (99.998%) were purchased from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (Changchun, China, http://www.ciac.jl.cn/). Stock standard solutions of La, Tb, and Lu were prepared from these oxides by dissolving in concentrated hydrochloric acid and diluting with distilled water. Working standard solutions were obtained by appropriate dilution of the stock standard solutions (1000 mg L–1). TA, with a structure characterized as five digallic acid units ester-linked to a glucose core, was supplied by Tianjin Guangfu chemical plant (Tianjin, China, http://guangfuchem.shuoyi.com/). NaOH and HCl were used to adjust solutions pH. All the reagents used were of analytical grade or better. Deionized water was used throughout the work.
Apparatus
The measurements of REEs were performed with an MPT-AES from Jilin University Little Swan Instruments Co., Ltd. (Changchun, China, http://kjy.jlu.edu.cn/). The MPT conditions were: microwave forward power 80 W, carrier gas (Ar) flow rate 1.0 L min–1, support gas (Ar) flow rate 0.6 L min–1, sheathing gas (O2) flow rate 1.0 L min–1, wavelength range 200 to 900 nm, entrance slit width 5 μm, CCD pixel elements 2048, optical resolution 0.065 nm, data transfer rate 13 ms, integration time 3 to 65000 ms. La, Tb, and Lu were determined at the wavelengths of 333.749 nm, 350.917 nm, and 261.541 nm, respectively. A UV-Vis spectrometer (TU-1800, Beijing Purkinje General Instruments Co., Ltd., China, http://www.pgeneral.com/) with 1 cm quartz cell was used for the spectrophotometric determination of TA (275 nm). A pHS-3 C digital pH meter (Rex Instruments Factory, Shanghai, China, http://www.lei-ci.com/) was employed for the pH measurements. Deionized water was prepared by the Milli-Q SP system (Millipore, Milford, MA, USA, http://www.millipore.com/).
Preparation of modified MWCNTs
Briefly, the MWCNTs were suspended in mixture of concentrated sulfuric acid and nitric acid (3:1 v/v), then sonicated for 2 h in an ultrasonic bath. The suspension was stirred at 55 oC for 7 h [38]. After cooled to room temperature, the oxidized MWCNTs (ox-MWCNTs) were washed repeatedly with deionized water until the residual acid was completely removed, and dried under vacuum for further use. The ox-MWCNTs (0.1 g) were dispersed in 30 mL 10 mg L–1 TA solutions and shaken continuously for a period of time at pH 3. Experiments were repeated at different pH values (1 to 6), contact time (60 to 600 min), and initial concentration of TA (5 to 100 mg L–1) to obtain the optimum modification method of oxidized MWCNTs with TA. The separated solid parts obtained after the adsorption step under the optimized conditions were washed, dried under vacuum and kept for the subsequent adsorption experiments.
Batch adsorption experiments
0.05 g TA-MWCNTs mixed with 10 mL REEs solution in batch reactors were shaken under controlled temperature of 293 K unless otherwise stated. Batch adsorption experiments were conducted to investigate REEs adsorption as a function of initial REEs concentration (5 to 50 mg L–1), aqueous pH (1 to 7), TA-MWCNTs dosage (0.02 to 0.2 g), and contact time (5 to 120 min). After reaching the sorption equilibrium, the aqueous phase was filtered with a syringe filter of 0.45 μm, and the final concentration of REEs was determined by MPT-AES. The adsorption capacity q e (mg/g TA-MWCNTs) was obtained as follows:
where q e is the amount of REEs adsorbed per unit amount of the adsorbent (mg g–1); C o and C are the initial and final concentrations (mg L–1) of REEs after adsorption, respectively; V is the volume of REEs solution (L); and m is the weight of TA-MWCNTs (g). In all batch experiments, average values were taken from triplicate measurements.
Elution studies
The elution studies were carried out in two steps. Firstly, stability of TA-MWCNTs; secondly, the recovery of the adsorbed REEs from TA-MWCNTs were determined in different concentrations of HCl. The concentrations of TA and REEs in the resulting solutions were determined by UV-Vis spectrometer and MPT-AES, respectively.
Results and discussion
Modification of method study
In order to achieve the best performance of TA onto ox-MWCNTs, the modification conditions have been optimized, such as contact time, pH, and TA concentration. Experimental data of effect of contact time, pH, and TA concentration are shown in Electronic Supplementary Material (Figs. S1–S3). Therefore, the optimized experimental conditions of modifying oxidized MWCNTs with TA are: contact time of 2 h, pH of 3, and TA concentration of 10 mg L–1. After the adsorption under such experimental parameters, the separated solid parts were washed, dried, and employed for the subsequent experiments. For the proper adsorption of REEs, the optimum amount of tannic acid immobilized per unit mass of oxidized MWCNTs was 3.45 mg g–1.
Characterization of adsorbents
The adsorbents were characterized using FT-IR spectra and transmission electron microscopy (TEM). The FT-IR spectra of MWCNTs, ox-MWCNTs, and TA-MWCNTs are studied and compared (Fig. S4 ESM). The HNO3-H2SO4 treatment produced carboxyl group on the surface of MWCNTs, as indicated by the presence of characteristic peaks at 3430 and 1716 cm–1 for stretching vibrations of O–H and C = O of the carboxyl group, respectively [38]. The intensity of the band representing –OH (3430 cm–1) stretching of alcoholic groups becomes stronger after MWCNTs compound with TA. 1535 cm–1was C–C stretching vibration from tetrasubstituted benzene rings [39], which is a main structure in the TA molecule. These FI-IR spectra confirm that MWCNTs have been successfully modified by tannic acid.
Figure 1 shows the TEM images of TA-MWCNTs. The nanotubes are curved with some open tips. The surfaces of MWCNTs are smooth and clean, and no obvious change of the surface structure and the framework of MWCNTs after modification with TA [38].
Adsorption of REEs with TA-MWCNTs
Effect of contact time on the adsorption of REEs with TA-MWCNTs
The effect of contact time on the adsorption of La, Tb, and Lu has been investigated when initial REEs concentrations are fixed at 40 mg L–1 (Fig. S5 ESM). The results demonstrate that the adsorption increases with increasing agitation time and attains equilibrium rapidly at about 50 min. This is obvious from the fact that a large number of vacant surface sites are available for adsorption in short time. A period of 60 min is chosen as a suitable contact time for La, Tb, and Lu.
Effect of sample pH on the adsorption of REEs with TA-MWCNTs
To determine the experimental conditions at which REEs are effectively adsorbed with TA-MWCNTs, the sorption studies have been carried out at different initial pH levels (Fig. S6 ESM). The oxidation of MWCNTs with concentrated HNO3 leads to the surface functionalization with oxygen containing groups, and the isoelectric point (IEP) of MWCNTs shift to lower pH values [40]. Furthermore, Lin et al. have demonstrated that Zeta potentials of the oxidation of CNTs in TA solutions do not significantly reduce [35]. In order to evaluate effect of pH, a series of sample solution are adjusted to a pH range of 1.5 to 7.0 and process according to recommended procedure. The adsorption capacity increases rapidly from 0.4 mg g–1 to approximately 6.0 mg g–1 with increasing pH from 1.5 to 4.0, and then gently increases from 4.0 to 7.0. At low pH range all metals are expected to exist predominantly in the Mn+ form. The concentration of H+ ions increases with decreasing pH, which competes with La, Tb, and Lu and makes the ion exchange reaction in the composite very difficult. The adsorption capacity rises in the pH range of 4.0 to 7.0. A possible reason may be that MWCNTs modified with TA having a number of functional groups is capable of forming chelates easily with REEs and thus improves the MWCNTs surface like humic substances [36]. The pH of 5.0 is selected in subsequent work.
Effect of adsorbent dosage on the adsorption of REEs with TA-MWCNTs
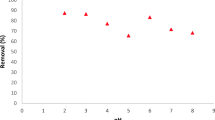
The amount of the adsorbent is an important parameter because it determines the adsorption capacity of an adsorbent for given initial concentration of the adsorbate. Figure 2 shows the effect of TA-WMCNTs amount on the adsorption capacity of La, Tb, and Lu in the dose range from 0.02 to 0.2 g, indicating that the adsorption capacity decreases almost linearly with increasing amount of adsorbent. This result indicates that the amount of La, Tb, and Lu adsorbed per unit weight of TA-MWCNTs decreases with an increase of adsorbent dosage. The decrease in unit adsorption with increasing adsorbent dosage is mainly due to the fact that the adsorption sites remain unsaturated during the adsorption reaction [28]. When the TA-MWCNTs dose is 0.1 g, the maximum adsorption capacities are 100% for REEs.
Sorption isotherms
In order to illustrate the interaction between the adsorbate and adsorbent, the obtained equilibrium isotherm adsorption data have been analyzed by the Langmuir and Freundlich isotherm models, which are most popular isotherm theories: [41]
where C e (mg L–1) is the equilibrium concentration of the solute, q e is the amount adsorbed at equilibrium (mg g–1), q max is the maximum adsorption at monolayer coverage (mg g–1), b is the adsorption equilibrium constant (L mg–1); x/m is the amount adsorbed per unit mass of the adsorbate, C e is the equilibrium concentration (mg L–1), K f (L mg–1) and n are the Freundlich isotherm constants.
The Langmuir and Freundlich constants are presented in Table 1. The calculated R 2 values indicate that both Langmuir and Freundlich isotherms model fit well. This implies that both monolayer sorption and heterogenous surface conditions exist under the experimental conditions used. Therefore, the sorption of REEs with TA-MWCNTs is complex, involving more than one mechanism.
Adsorption capacity of TA-MWCNTs has been evaluated to be 5.35, 8.55, and 3.97 mg g–1 for La, Tb, and Lu, respectively. Such adsorption capacities are comparable to those reported in other papers. The adsorption capacities of REEs with various adsorbent are summarized in Table 2. For instance, Jain et al. [20] have reported that the adsorption capacity of XAD-4 functionalized with o-vanillinsemicarbazone is 2.3 mg g–1 for La. Hang et al. [7] have employed nanometer TiO2 as a new type of adsorption material to separate and concentrate trace REEs. The adsorption capacity of nanometer TiO2 for REEs is in the range of 11.3 ~ 21.6 mg g–1. However, it should be noted that compared with biomasses [25] or magnetic materials [28], the adsorption capacity of TA-MWCNTs is low. A possible strategy to enhance the adsorption capacity may be achieved by using MWCNTs with smaller sizes or exploring more effective modification methods on MWCNTs.
Sorption kinetics
In order to investigate the rate controlling step of adsorption process, the kinetic adsorption experiments of La, Tb and Lu on TA-MWCNTs are performed. After 60 min contact time for La, Tb and Lu, the adsorption equilibrium is reached in a solid solution (Fig. S5, ESM). The pseudo-first-order and pseudo-second-order kinetic model have been employed to fit the kinetic adsorption data [42–44]. The pseudo-first-order and pseudo-second-order kinetic model can be expressed as follows:
where q t and q e (mg g–1) are the amounts of metal ions adsorbed at any time t (min) and at equilibrium, respectively, k 1 (min–1) and k 2 (g mg–1 min–1) are the adsorption rate constant of pseudo-first-order and pseudo-second-order adsorption.
For the studied concentration, the rate constants (k 1 and k 2) and theoretical equilibrium sorption capacities, q e are calculated from the slope and the intercept of the linear plot of the pseudo-first-order and pseudo-second-order kinetic models. It is important to note that for a pseudo-first-order model, the correlation coefficient is always less than 0.90, which is indicative of a bad correlation (Fig. S7 ESM). In contrast, the application of a pseudo-second-order model leads to much better regression coefficients, all greater than 0.99 (Fig. 3). Thus, the pseudo-second-order model is suitable to model the adsorption curves of La, Tb, and Lu with TA-MWCNTs. The values of k 2 and q e are calculated from the slope and intercept, respectively, of a liner plot of t/q t versus t and are given in Table S1, ESM.
Recovery and reusability of TA-MWCNTs
For an effective recycling process, adsorbed REEs should be easily desorbed without destroying the adsorbent materials under suitable conditions. Firstly, the stability of TA-MWCNTs has been investigated when HCl is employed as the eluent. When 1.0 mol L–1 HCl is employed to desorb TA, the recovery reaches no more than 5.0%. Secondly, 0.1 g TA-MWCNTs have been used to adsorb La, Tb, and Lu (40 mg L–1), and then the quantitative recovery of La, Tb, and Lu is performed using 20 mL various concentrations of HCl. As can be seen in Fig. 4, 1.0 mol L–1 HCl is sufficient for complete elution of La, Tb, and Lu from TA-MWCNTs and the recovery is more than 95%. Therefore, 1.0 mol L–1 HCl solution is selected for the elution of La, Tb, and Lu in the experiments. The adsorption process has been repeated by using the regenerated adsorbent. It can be found that after three cycles there is no significantly change in the sorption capacity of TA-MWCNTs. Thus, TA-MWCNTs are regenerable and can be used several times.
Thermodynamic parameters
The effect of temperature on the adsorption of La, Tb, and Lu with TA-MWCNTs has been studied at different temperatures from 293 K to 323 K. Thermodynamic parameters associated with the sorption viz., free energy change (ΔG), enthalpy change (ΔH), entropy change (ΔS) were calculated as follows [45]:
Where D is distribution ratios, b is the adsorption equilibrium constant (L mg–1) shown in Eq. 2. The values of ΔH, ΔG and ΔS are calculated and summarized in Table S2, ESM. ΔH values are all positive, i.e., increasing temperature is advantageous for the reactions, indicating that the three systems are all endothermically driven. Negative values of ΔG suggest that the sorption process is spontaneous with high preference of REEs for TA-MWCNTs. The positive entropy changes indicate that the degree of freedom increases at the solid-liquid interface during the adsorption of REEs with TA-MWCNTs.
Conclusions
In this research, the preparation and application of a sorbent based on the modification of MWCNTs with TA have been demonstrated. The preparation of TA-MWCNTs is proved to be relatively simple and rapid. TA-MWCNTs are effective adsorbents for the removal of REEs. The influence of various experimental parameters, contact time, initial solution pH, and adsorbent dose, on the adsorption of REEs has been investigated. Equilibrium data have been fitted to Langmuir isotherms and Freundlich isotherms, indicating that both monolayer sorption and heterogenous surface conditions exist under the experimental conditions used. The sorption process is found to be endothermic in nature. The rate of sorption follows the pseudo-second-order kinetic model.
References
Li Y, Hu B (2010) Cloud point extraction with/without chelating agent on-line coupled with inductively coupled plasma optical emission spectrometry for the determination of trace rare earth elements in biological samples. J Hazard Mater 174:534
Fujimori F, Hayashi T, Inagaki K, Haraguchi H (1999) Determination of lanthanum and rare earth elements in bovine whole blood reference material by ICP-MS after coprecipitation preconcentration with hemeiron as coprecipitant. Fresenius J Anal Chem 363:277
Tong SS, Song NZ, Jia Q, Zhou WH, Liao WP (2009) Solvent extraction of rare earths from chloride medium with mixtures of 1-phenyl-3-methyl-4-benzoyl-pyrazalone-5 and sec-octylphenoxyacetic acid. Sep Purif Technol 69:97
Sun XQ, Peng B, Ji Y, Chen J, Li DQ (2008) The solid-liquid extraction of yttrium from rare earths by solvent (ionic liquid) impreganated resin coupled with complexing method. Sep Purif Technol 63:61
Jia Q, Kong XF, Zhou WH, Bi LH (2008) Flow injection on-line preconcentration with an ion-exchange resin coupled with microwave plasma torch-atomic emission spectrometry for the determination of trace rare earth elements. Microchem J 89:82
Rauf MA, Hussain MT, Hasany SM (1993) Adsorption of europium on manganese dioxide from binary mixtures of aqueous sulfuric acid and methanol. Sep Sci Technol 28:2237
Hang YP, Qin YC, Jiang ZC, Hu B (2003) Direct analysis of trace rare earth elements through nanometer-size titanium dioxide separation/concentration and fluorination assisted ETV-ICP-AES with slurry sampling. Chem J Chin Univ 24:1980
Quan GR, Yang LH, Pu XL, Hu B, Jiang ZC, Peng TY (2004) Study on adsorption behaviors of rare earth ions on nanometer Al2O3 powder by ICP-AES. J Anal Sci 20:337
Liang P, Cao J, Liu R, Liu Y (2007) Determination of trace rare earth elements by inductively coupled plasma optical emission spectrometry after preconcentration with immobilized nanometer titanium dioxide. Microchim Acta 159:35
Pasinli T, Eroglu AE, Shahwan T (2005) Preconcentration and atomic spectrometric determination of rare earth elements (REEs) in natural water samples by inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 547:42
Naik PW, Dhami PS, Misra SK, Jambunathan U, Mathur JN (2003) Use of organophosphorus extractants impregnated on silica gel for the extraction chromatographic separation of minor actinides from high level waste solutions. J Radioanal Nucl Chem 257:327
Bou-Maroun E, Goetz-Grandmont GJ, Boos A (2007) Solid-liquid extraction of Lanthanum(III), europium(III), and lutetium(III) by acyl-hydroxypyrazoles entrapped in mesostructured silica. Sep Sci Technol 42:1913
Chowdhury P, Pandit SK, Mandal B (2008) Solid phase extraction of cerium(IV) with crosslinked poly(acrylic acid) coated on silica gel. Indian J Chem 47A:1528
Zhang AY, Wei YZ, Kumagai MK (2007) Separation of minor actinides and rare earths from a simulated high activity liquid waste by two macroporous silica-based polymeric composites. Sep Sci Technol 42:2235
Liang P, Fa WJ (2005) Determination of La, Eu and Yb in water samples by inductively coupled plasma atomic emission spectrometry after solid phase extraction of their 1-phenyl-3-methyl-4-benzoylpyrazol-5-one complexes on silica gel column. Microchim Acta 150:15
Gad HMH, Awwad NS (2007) Factors affecting on the sorption/desorption of Eu(III) using activated carbon. Sep Sci Technol 42:3657
Lee GS, Uchikoshi M, Mimura K, Isshiki M (2009) Distribution coefficients of La, Ce, Pr, Nd, and Sm on Cyanex 923-, D2EHPA-, and PC88A-impregnated resins. Sep Purif Technol 67:79
Kim JS, Han C, Wee JH (2006) Effect of polyvinyl alcohol on rare earths (Gd and Tb) separation by extraction resin. Talanta 68:963
Ansari SA, Pathak PN, Husain M, Prasad AK, Parmar VS, Manchanda VK (2006) Extraction chromatographic studies of metal ions using N, N, N', N'-tetraoctyl diglycolamide as the stationary phase. Talanta 68:1273
Jain VK, Handa A, Sait SS, Shrivastav P, Agrawal YK (2001) Pre-concentration, separation and trace determination of lanthanum(III), cerium(III), thorium(IV) and uranium(VI) on polymer supported o-vanillinsemicarbazone. Anal Chim Acta 429:237
Jelinek L, Wei YZ, Arai T, Kumagai M (2007) Selective Eu(III) electro-reduction and subsequent separation of Eu(II) from rare earths(III) via HDEHP impregnated resin. Solvent Extr Ion Exch 25:503
Draye M, Czerwinski KR, Favre-Reguillon A, Foos J, Guy A, Lemaire M (2000) Selective separation of lanthanides with phenolic resins: extraction behavior and thermal stability. Sep Sci Technol 35:1117
Choi KS, Lee CH, Kim JG, Kim WH, Kang JG (2007) Separating Ag, B, Cd, Dy, Eu, and Sm in a Gd matrix using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester extraction chromatography for ICP-AES analysis. Talanta 71:662
Dave SR, Kaur H, Menon SK (2010) Selective solid-phase extraction of rare earth elements by the chemically modified Amberlite XAD-4 resin with azacrown ether. React Funct Polym 70:692
Diniz V, Volesky B (2005) Biosorption of La, Eu and Yb using Sargassum biomass. Wat Res 39:239
Li JP, Song LM, Zhang SJ (2002) Rare earth metal ion adsorption capacity on cross-linked magnet chitosan. J Rare Earth 20:219
Shan XQ, Lian J, Wen B (2002) Effect of organic acids on adsorption and desorption of rare earth elements. Chemosphere 47:701
Wu DB, Zhao J, Zhang L, Wu QS, Yang YH (2010) Lanthanum adsorption using iron oxide loaded calcium alginate beads. Hydrometallurgy 101:76
Zhang L, Ding SD, Yang T, Zheng GC (2009) Adsorption behavior of rare earth elements using polyethyleneglycol (phosphomolybdate and tungstate) heteropolyacid sorbents in nitric solution. Hydrometallurgy 99:109
Fan QH, Shao DD, Hu J, Chen CL, Wu WS, Wang XK (2009) Adsorption of humic acid and Eu(III) to multi-walled carbon nanotubes: effect of pH, ionic strength and counterion effect. Radiochim Acta 97:141
Chen SZ, Liu C, Lu DB, Zhu L (2009) Determination of trace rare earth elements by ICP-MS after on-line column preconcentration and separation with single-wall carbon nanotubes. At Spectrosc 30:20
Tong SS, Shi YF, Song NZ, Liu W, Jia Q, Zhou WH (2010) Adsorption of rare earths with multi-walled carbon nanotubes. Chin J Appl Chem 27:944
Wan HJ, Zou QL, Yan R, Zhao FQ, Zeng BZ (2007) Electrochemistry and voltammetric determination of tannic acid on a single-wall carbon nanotube-coated glassy carbon electrode. Microchim Acta 159:109
Lin DH, Liu N, Yang K, Zhu LZ, Xu Y, Xing BS (2009) The effect of ionic strength and pH on the stability of tannic acid-facilitated carbon nanotube suspensions. Carbon 47:2875
Lin DH, Xing BS (2008) Tannic acid adsorption and its role for stabilizing carbon nanotube suspensions. Environ Sci Technol 42:5917
Üçer A, Uyanık A, Aygün ŞF (2006) Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Sep Purif Technol 47:113
Üçer A, Uyanık A, Çay S, Özkan Y (2005) Immobilisation of tannic acid onto activated carbon to improve Fe(III) adsorption. Sep Purif Technol 44:11
Liu Y, Li Y, Yan XP (2008) Preparation, characterization, and application of L-Cysteine functionalized multiwalled carbon nanotubes as a selective sorbent for separation and preconcentration of heavy metals. Adv Funct Mater 18:1536
Simons WW (1978) The Sadtler handbook of infrared spectra. Sadtler Research Laboraries, Philadelphia
Li YH, Wang SG, Luan ZK, Ding J, Xu CL, Wu DH (2003) Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 41:1057
Qu R, Sun C, Wang M, Ji C, Xu Q, Zhang Y, Wang C, Chen H, Yin P (2009) Adsorption of Au(III) from aqueous solution using cotton fiber/chitosan composite adsorbents. Hydrometallurgy 100:65
Gupta V, Gupat M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Wat Res 35:1125
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Wat Res 34:735
Weng CH, Huang CP (2004) Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids Surf A 247:137
Acknowledgment
Financial support was provided by Basic Scientific Research Fund of Jilin University (2008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 71 kb)
Rights and permissions
About this article
Cite this article
Tong, S., Zhao, S., Zhou, W. et al. Modification of multi-walled carbon nanotubes with tannic acid for the adsorption of La, Tb and Lu ions. Microchim Acta 174, 257–264 (2011). https://doi.org/10.1007/s00604-011-0622-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0622-3