Abstract
A sensitive and selective method for the speciation of Cr(III) and Cr(VI) in water samples was developed. It is based on the selective binding of the complex formed between Cr(III) and 4-(2-pyridylazo)resorcinol adsorbed on a cross-linked polymer modified with β-cyclodextrin and placed in a micro-column. Graphite furnace atomic absorption spectrometry (GFAAS) was used to quantify chromium. Cr(VI) ion is not adsorbed but remains in the aqueous sample phase. Thus, an in-situ separation of Cr(VI) and Cr(III) is accomplished. The concentration of Cr (VI) was calculated by subtracting the value for Cr(III) from that for total chromium. Under optimum conditions, the limit of detection of Cr(III) is 0.056 μg L−1, and the linear range is from 2.0 to 160.0 μg L−1. The relative standard deviation is 2.5% (n = 3, at 30.0 μg L−1). The preconcentration factor is 25. The method was applied to the speciation of chromium in water samples, and recoveries in spiked real samples range from 101.9% to 104.5%. A reference water sample (GBW(E)080642) also was analyzed, and the results were in good agreement with the certified values.

The quantitative adsorption (≥90%) on the β-CDCP for Cr(III) was found in the range of the pH 5.5–6.0, whereas the adsorption efficiency for Cr(VI) at this pH range was rather low.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the environment chromium occurs mainly in two different oxidation states, chromium(III) and chromium(VI). Chromium(III) is considered one of the essential elements for living organisms, whereas chromium(VI) is toxic to human, and was found to be carcinogenic. In recent years, chromium enters the environment from the discharge of steel, electroplating, tanning industries etc., leading to serious problems and hazardous risks for human health. The analysis of total chromium cannot be utilized to evaluate the environmental impact. For speciation analysis in environmental studies, the determination of redox species (Cr(VI)/Cr(III)) is very important. Therefore, the development of methods for the determination of the two species of Cr is required [1–4].
The direct determination of noxious trace elements in real samples may not be possible because of low concentrations and/or matrix interferences, so a separation/preconcentration technique were necessary [5]. Various separation/analysis methods have been employed to speciation analysis of Cr(III) and Cr(VI), such as liquid–liquid extraction-GFAAS [6], coprecipitation-FAAS [7, 8], cloud point extraction-FAAS [9], cloud point extraction-HPLC [10], cloud point extraction-ETAAS [11], ion exchange-FAAS [12], and solid phase extraction-FAAS [3, 13, 14], solid phase extraction-ICP-AES [15], etc. In our previous publication, the speciation of chromium by cloud point extraction-ETAAS [11] and by in situ separation and sequential determination with electrothermal vaporization inductively coupled plasma atomic emission spectrometry [16] were researched.
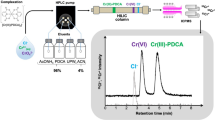
Solid phase extraction (SPE) technique is widely applied to preconcentration and speciation of trace metals because of its advantages of high enrichment factor, high recovery, rapid phase separation, low consumption of organic solvents and the ability of combination with different detection techniques in the form of on-line or off-line mode [17]. Nowadays, many adsorption materials, such as activated carbon [18], alumina [19, 20], chelating resins [21, 22] and nanometer material [15] were employed on the speciation studies of chromium. β-CDCP synthesized by the reaction of β-CD and cross-linked agent[23] was a spherical or grainy solid subject, which retained the inclusion property of β-CD. β-CDCP as a solid phase extraction material had been applied to separation/preconcentration the trace metal element [23, 24]. But β-CDCP-micro-column used as SPE material to speciation of Cr(III) and Cr(VI) seem to be lacking.
The present work aimed at investigating the separation Cr(III)/Cr(VI), the complex of Cr(III) with 4-(2-pyridylazo)-resorcinol (PAR) could been adsorbed on β-CDCP micro-column, whereas the Cr(VI) remained in aqueous phase. Thus, an in situ separation of Cr(VI) and Cr(III) could be realized. Cr(VI) was calculated by subtracting of Cr(III) from the total chromium. Total chromium was determined after reducing Cr(VI) to Cr(III). The developed method was applied to the speciation analysis of Cr(VI) to Cr(III) in tap water with satisfactory results.
Experimental
Apparatus
A ZEEnit 700 graphite furnace atomic absorption spectrometer (analytikjena, Germany) was used for the determination of Cr(III) and Cr(VI). The operating conditions for GFAAS are listed in Table 1. UV-2550 spectrophotometer (Shimadzu, Japan) and digital constant temperature water-bath (Guohua Limited Company, China) were used. The pH values were measured with a pHS-25 pH-meter (Shanghai, China). A BT-001 peristaltic pump (Shanghai Zhisun Instrument Factory, China) was used in separation and preconcentration process. A self-made PTFE micro-column (20 mm × 2.0 mm i.d.) was packed with β-CDCP in the manifold for separation/preconcentration.
Reagents
All chemicals used were of analytical grade. Cr(III) stock standard solution (1.0 mg L−1) was prepared by dissolution of 0.1 g metallic chromium (Tokyo, Japan 5N) in concentrated hydrochloric acid and dilution with water to 100 mL. Cr(VI) stock standard (1.0 mg L−1) solution was prepared by dissolution of 0.2830 g K2Cr2O7 (Shanghai Reagent Factory, Shanghai, China) in water and dilution to 100 mL. 4-(2-Pyridylazo) resorcinol (PAR) solution of 1.0 × 10−3 mol L−1 was prepared from regent grade PAR (A.R., Shanghai Reagent Factory, Shanghai, China) by dissolving 21.5 mg in 100 mL of alcohol. The required pH adjustments were made by the CH3COOH/CH3COONH4 buffer solutions. The reduction of Cr(VI) to Cr(III) was carried out using hydroxylamine hydrochloride (Nantong, China) as the reducing agent.
Column preparation
Fifty milligrams of β-CDCP was introduced into a PTFE micro-column (20 mm × 2.0 mm i.d.) plugged with a small portion of glass-wool at both ends. The column was washed thoroughly with distilled water and then preconditioned to the desired pH with CH3COOH/CH3COONH4 buffer before passing chromium ions containing solutions.
General preconcentration procedure for Cr(III)
The amount of Cr(III) (1.5 mL, 100.0 μg L−1), 0.25 mL PAR (1.0 × 10−3 mol L−1, ethanol) was mixed, controlling the appropriate acidity. The column was preconditioned by passing the buffer solution with pH 6.0 through the micro-column and then the standard solutions were passed through the column at a flow rate of 1.0 mL min−1. The Cr(III)-PAR complex retained on β-CDCP was eluted with 1.0 mL of 1.0 mol L−1 HCl solution. The concentration of the chromium was determined by GFAAS.
Reduction of Cr(VI) to Cr(III) and determination of total chromium
Reduction of Cr(VI) ions to Cr(III) was performed by using the procedure given in the literature [2, 25]. 1.0 mL of 2.0 mol L−1 HCl and then 0.5 mL of 5% (w/v) hydroxylamine hydrochloride were added to 3.0 mL of sample solution containing 150 ng Cr(III) and 150 ng Cr(VI), respectively. The solution was left at room temperature for 45 min. After the reduction of Cr(VI) to Cr(III), the described preconcentration procedure was applied and total chromium was determined by GFAAS. The Cr(VI) concentration was calculated by substracting the Cr(III) concentration from total chromium concentration.
Results and discussion
Spectral characteristics
The UV–vis spectra of Cr(III)-PAR and Cr(VI)-PAR at pH 6.0 were shown in Fig. 1. As could be seen that the λmax of Cr(III)-PAR complex was at 542 nm. On the contrary, there was no absorbance for Cr(VI)/PAR due to no complexation between Cr(VI) and PAR.
Optimization of adsorption conditions
Effect of pH
The effect of pH (4.0–7.5) on the adsorption of Cr(III) and Cr(VI) on the β-CDCP column was studied (Fig. 2). It was showed that the quantitative adsorption (≥90%) for Cr(III) was found in the range of the pH 5.5–6.0, whereas the adsorption efficiency for Cr(VI) at this pH range was rather low (<10%). For all subsequent experiments, pH 6.0 was selected as optimal.
Effect of flow rate of sample solution
The flow rate of sample solution affected the adsorption of complex and the duration of analysis. The flow rate (1.0–2.0 mL min−1, Fig. 3) of sample solution was examined under the optimum condition. It was found that the adsorption of Cr(III)-PAR was quantitative when the flow rate <1.25 mL min−1. So, 1.0 mL min−1 was chosen as the sample flow rate in subsequent experiments.
Effect of the size of β-CDCP
The effect of the size of β-CDCP (100–300 mesh) on the adsorption efficiency was investigated (Fig. 4). With the decrease of the size of β-CDCP, the adsorption efficiency of Cr(III)-PAR complex on β-CDCP increased. When the mesh size of β-CDCP was larger than 200 mesh, Cr(III)-PAR could be quantitatively adsorbed. So, the size of 300 mesh was chosen.
Effect of the sample volume
The effect of the sample volumes on the adsorption efficiency of Cr(III)-PAR on β-CDCP was investigated. For this purpose, 5.0–45.0 mL volumes of sample solutions containing 150.0 ng Cr(III) were passed through the column under the optimum conditions. The results were shown that the adsorption efficiency of Cr(III) was greater than 90% in the volume of sample 5.0–25 mL, when the volume of sample was larger than 25.0 mL, the adsorption efficiency is lesser than 90%. In this work, the sample volume of 25.0 mL was adopted. The preconcentration factor was 25 (the quotient of volume before absorption and after elution).
Adsorption capacity
To determine the adsorption capacity of the β-CDCP (maximum amount of Cr(III) retained from 1.0 g of β-CDCP), 15.0 mL sample solutions(Cr(III) concentrations from 5.0 to 200.0 μg L−1) was adjusted to pH 6.0 with CH3COOH/CH3COONH4 buffer, and the separation and preconcentration procedure described above was applied. The amount of Cr(III) adsorbed at each concentration level was determined. The breakthrough curve for Cr(III) was gained by plotting the concentration (μg L−1) of Cr(III) solution versus the micrograms of Cr(III) adsorbed per gram β-CDCP. When the concentration of Cr(III) reached 120.0 μg L−1, the retention capacity arrived at its maximum value. A adsorption capacity of the polymer was calculated to be 32.5 μg g−1.
Effect of HCl concentration and volume on desorption efficiency
For the elution of adsorbed Cr(III)-PAR on β-CDCP, diluted hydrochloric acid solutions at different volumes were used as an eluent. The desorption efficiency of Cr(III)-PAR with 0.25–3.0 mol L−1 hydrochloric acid solutions and 0.5–3.0 mL of HCl was investigated. So optimum volume of HCl (1.0 mol L−1) solution chosen for this work was 1.0 mL.
Column reuse
The stability and potential regeneration of β-cyclodextrin-cross-linked polymer micro-column were investigated. The β-cyclodextrin-cross-linked polymer micro-column was reproduced 6 times as section 2.4 and its adsorption efficiency for Cr(III)-PAR is greater than 90%. It demonstrated that β-CDCP exhibited good reversibility and reproducibility, which was associated with the fact that the inclusion interaction between β-CDCP and Cr(III)-PAR was a reversible process.
Effect of interfering ions
The effect of various interfering ions found in water samples on the determination of Cr(III) was studied. With a relative error of less than ±5%, the tolerance limits for the potentially interfering substances were listed in Table 2. These results show that Cr(III) could be determined quantitatively in water samples.
Analytical parameters
The calibration graph for Cr (III) was linear 2.0–160.0 μg L−1, the corresponding coefficient of correlation was r = 0.9998. The relative standard deviation (R.S.D) was 2.47% (n = 3,c = 30.0 μg L−1). According to the dentition of IUPAC, the detection limit (3σ) of this method was 0.056 μg L−1. The preconcentration factor was 25.
Table 3 compares the characteristic data of the present method with those reported in literatures. Generally, the enrichment factor obtained by the present method is comparable to those reported method, and the detection limit is better than them.
Accuracy and applications of the method
For the validation of the method, a standard reference material (GBW(E) 080642) water sample after 1,000-fold dilution with deionized double distilled water was analyzed and the analytical results were in good agreement with the certified values (Table 4). The speciation procedure was applied to the speciation of chromium in tap water and the analytical results along with the recovery are given in Table 5. As could be seen, recoveries in the range of 98.6–104.8% were obtained.
Conclusion
A new method is used for the speciation of chromium by SPE combined with GFAAS due to the formation of Cr(III)-PAR in this paper. Cr(III)-PAR was quantitatively retained on polymer β-CDCP at pH 6.0, while Cr(VI) remained in the solution. The method is characterized with simplicity, rapidity, selectivity, safety, low cost and high preconcentration factor, and is suitable for the determination of Cr(III) and Cr(VI) in environmental water samples.
References
Barnowski C, Jakubowski N, Stuewer D, Broekaert JAC (1997) Speciation of chromium by direct coupling of ion exchange chromatography with inductively coupled plasma mass spectrometry. J Anal At Spectrom 12:1155
Tokalıoğlu Ş, Arsav S, Delibaş A, Soykan C (2009) Indirect speciation of Cr(III) and Cr(VI) in water samples by selective separation and preconcentration on a newly synthesized chelating resin. Anal Chim Acta 645:36
Tunçeli A, Türker AR (2002) Speciation of Cr(III) and Cr(VI) in water after preconcentration of its 1,5-diphenylcarbazone complex on amberlite XAD-16 resin and determination by FAAS. Talanta 57:1199
Yalçin S, Apak R (2004) Chromium(III, VI) speciation analysis with preconcentration on a maleic acid-functionalized XAD sorbent. Anal Chim Acta 505:25
Sperling M, Yin XF, Welz B (1992) Differential determination of chromium(VI) and totalchromium in natural water using flow injection on-line separation and preconcentration electrothermal atomic absorption spectrometry. Analyst 117:629
Béni Á, Karosi R, Posta J (2007) Speciation of hexavalent chromium in waters by liquid–liquid extraction and GFAAS determination. Microchem J 85:103
Karatepe A, Korkmaz E, Soylak M, Elci L (2010) Development of a coprecipitation system for the speciation/preconcentration of chromium in tap waters. J Hazard Mater 173:433
Uluozlu OD, Tuzen M, Soylak M (2009) Speciation and separation of Cr(VI) and Cr(III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem Toxicol 47:2601
Sun ZM, Liang P (2008) Determination of Cr(III) and total chromium in water samples by cloud point extraction and flame atomic absorption spectrometry. Microchim Acta 162:121
Tang AN, Jiang DQ, Jiang Y, Wang SW, Yan XP (2004) Cloud point extraction for high-performance liquid chromatographic speciation of Cr(III) and Cr(VI) in aqueous solutions. J Chromatogr A 1036:183
Zhu XS, Hu B, Jiang ZC, Li MF (2005) Cloud point extraction for speciation of chromium in water samples by electrothermal atomic absorption spectrometry. Water Res 39:589
Sule PA, Ingle JD Jr (1996) Determination of the speciation of chromium with an automated two-column ion-exchange system. Anal Chim Acta 326:85
Narin I, Kars A, Soylak M (2008) A novel solid phase extraction procedure on Amberlite XAD-1180 for speciation of Cr(III), Cr(VI) and total chromium in environmental and pharmaceutical samples. J Hazard Mater 150:453
Saygi KO, Tuzen M, Soylak M, Elci L (2008) Chromium speciation by solid phase extraction on Dowex M 4195 chelating resin and determination by atomic absorption spectrometry. J Hazard Mater 153:1009
Liang P, Shi TQ, Lu HB, Jiang ZC, Hu B (2003) Speciation of Cr(III) and Cr(VI) by nanometer titanium dioxide micro-column and inductively coupled plasma atomic emission spectrometry. Spectrochim Acta B 58:1709
Zhu XS, Hu B, Jiang ZC, Wu YL, Xiong S (2002) Speciation of chromium(III) and chromium(VI) by in situ separation and sequential determination with electrothermal vaporization inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 471:121
Fritz JS (1999) Analytical solid-phase extraction. Wiley-VCH, New York
Gil RA, Cerutti S, Gásquez JA, Olsina RA, Martinez LD (2006) Preconcentration and speciation of chromium in drinking water samples by coupling of on-line sorption on activated carbon to ETAAS determination. Talanta 68:1065
Marqués MJ, Rubio AM, Salvador A, de la Guardia M (2001) Chromium speciation using activated alumina microcolumns and sequential injection analysis-flame atomic absorption spectrometry. Talanta 53:1229
Manzoori JL, Sorouraddin MH, Shemirani F (1995) Chromium speciation by a surfactant-coated alumina microcolumn using electrothermal atomic absorption spectrometry. Talanta 42:1151
Hassanien MM, Kenawy IM, El-Menshawy AM, El-Asmy AA (2008) A novel method for speciation of Cr(III) and Cr(VI) and individual determination using Duolite C20 modified with active hydrazone. J Hazard Mater 158:170
Pramanik S, Dey S, Chattopadhyay P (2007) A new chelating resin containing azophenolcarboxylate functionality: synthesis, characterization and application to chromium speciation in wastewater. Anal Chim Acta 584:469
Zhu XS, Wu M, Gu Y (2009) β-Cyclodextrin-cross-linked polymer as solid phase extraction material coupled with inductively coupled plasma mass spectrometry for the analysis of trace Co(II). Talanta 78:565
Zhu XS, Wu M, Sun J, Zhang XF (2008) β-Cyclodextrin-cross-linked polymer as solid phase extraction material coupled graphite furnace atomic absorption spectrometry for separation/Analysis of trace copper. Anal Lett 41:2186
Sumida T, Ikenoue T, Hamada K, Sabarudin A, Oshima M, Motomizu S (2005) On-line preconcentration using dual mini-columns for the speciation of chromium(III) and chromium(VI) and its application to water samples as studied by inductively coupled plasma-atomic emission spectrometry. Talanta 68:388
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (20875082) and the Foundation of Excellence Science and Technology Invention Team in the Yangzhou University
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, Y., Zhu, X. Speciation of Cr(III) and Cr(VI) ions using a β-cyclodextrin-crosslinked polymer micro-column and graphite furnace atomic absorption spectrometry. Microchim Acta 173, 433–438 (2011). https://doi.org/10.1007/s00604-011-0578-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0578-3