Abstract
An electrochemiluminescence-based immunoassay using quantum dots (QDs) as labels for the carcinoembryonic antigen (CEA) was developed using an electrode modified with leafs of nanoporous gold. CEA was initially immobilized on the electrode via a sandwich immunoreaction, and then CdTe quantum dots capped with thioglycolic acid were used to label the second antibody. The intensity of the ECL of the QDs reflects the quantity of CEA immobilized on the electrode. Thus, in the presence of dithiopersulfate as the coreactant, the ECL serves as the signal for the determination of CEA. The intensity of the electroluminescence (ECL) of the electrode was about 5.5-fold higher than that obtained with a bare gold electrode. The relation between ECL intensity and CEA concentration is linear in the range from 0.05 to 200 ng.mL-1, and the detection limit is 0.01 ng.mL-1. The method has the advantages of high sensitivity, good reproducibility and long-term stability, and paves a new avenue for applying quantum dots in ECL-based bioassays.

Electrochemiluminescence Immunoassay Based on CdTe Quantun Dots as labels at Nanoporous Gold Leaf electrode
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrochemiluminescence (ECL), as a process of generating electrochemical species and forming excited states that emit light, has been widely used in immunoassay due to its versatility, simplicity, stability and high sensitivity [1–4]. Up to now, a variety of ECL luminophores including inorganic [5, 6] and organic luminophores [7] have been discovered and extensively applied in immunoassay [8]. Recently, quantum dots (QDs), as a new kind of ECL luminophores, have begun to be applied in fabricating ECL biosensor. Table 1 lists some of the ECL immunoassays by the use of quantum dots. These assays are all based on the quench effect of immunoreaction on the ECL of QDs. The target antigen was measured through the decrease of ECL intensity resulting from the specific binding of antibody to antigen. Besides, multiple amplification strategies such as combining QDs with gold nanoparticles [9] or carbon nanotubes [10, 11] and self-assembling gold nanoparticles on electrode [12, 13] have been adopted to increase the sensitivity. However, the process of preparing QDs-nanoparticle composites and fabricating nanoparticle modified electrode is complicated, inconvenient and time-consuming.
Recently, Ding’s group [14–16] have reported a simple dealloying route to make ultra-thin nanoporous gold leaf (NPGL) that is free-standing, highly conductive, large surface area, and easy attachment to a variety of substrates. The nanoporous gold leaf can be used to enhance the electrochemiluminescence of quantum dots because of its porous structure which can provide more binding sites and reduce electron injection barrier to quantum dots. What is more, the fabricating of nanoporous gold leaf modified electrode is very simple and rapid. For these reasons, here, we proposed a novel immunoassay based on quantum dots ECL with NPGL amplification. Different from the conventional ECL immunoassay, we make QDs as lables and the ECL intensity would increase gradually with the increasing concentration of target antigen.
Carcinoembryonic antigen (CEA), as one kind of glycoprotein with a molecular weight of about 200 kDa, has been widely used as a tumor marker for clinical diagnosis such as colon cancer, lung cancer and ovarian carcinoma [17–20]. So the detection of CEA is of particular importance in clinical tumor diagnosis and even prognosis of original liver carcinoma. Many immunoassay techniques including radioimmunoassay [21], enzyme-linked immunosorbent [22] assay and fluorescence measurement [23] have been used for CEA detection. Recently, electrochemical immunoassay with the advantages of simple, inexpensive, rapid analysis and high sensitivity has attracted growing interest [24–26]. However, the reports concering ECL detection of CEA are relatively scarce. In this paper, we chose CEA as target antigen and realized the ECL immunoassay through QDs labling with nanoporous amplification technique. In this assay, CEA was firstly immobilized via a sandwich immunoreaction on the electrode modified with nanoporous gold leafs. After the specific binding of CEA with second antibody labeled by QDs, the ECL signals from QDs could directly reflect the concentration of CEA. This method combined the advantages of electrochemical immunoassay and quantum dots ECL and would have a great potential in the clinical protein detection.
Experimental
Chemicals and materials
CEA (Ag, 200 kDa), monoclonal antibody to CEA (Ab1 and Ab2) were obtained from Shanghai Linc-Bio Science Co. Ltd. (Shanghai, China, http://lincbio.diytrade.com), Tellurium powder and potassium borohydride from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), cadmium chloride from Alfa Aesar China Ltd. (Tianjin, China), bovine serum albumin (BSA), thioglycolic acid from Sigma (St. Louis, MO, USA, http://www.sigmaaldrich.com), N-hydroxysuccinimide (NHS) from Huifeng Chemical Industry Ltd. (Weinan, China), 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide hydrochloride (EDC) from Shanghai Medpep Co. Ltd. (Shanghai, China, http://www.medpep.com), dithiopersulfate (K2S2O8) from Tianjin Dongfang Chemical Plant (Tianjin, China), The phosphate buffered solution consisted of 0.15 mol.L-1 NaCl, 7.6×10-3 mol.L-1 NaH2PO4 and 2.4×10-3 mol.L-1 Na2HPO4 (pH 7.4). Thioglycolic acid capped CdTe QDs were synthesized as described in previous report [27] and the size estimated from the empirical equations [28] was about 2.5 nm. All aqueous solutions were prepared with doubly distilled water.
Nanoporous gold leafs were offered by Ding’s group of Shandong University. The NPGL electrode was prepared by attaching the nanoporous gold leafs onto a 6-mm diameter Au disk electrode, and cured for 20 min at 120 °C in an oven. Before the fabrication, the Au disk electrode was polished with 0.05 μm γ-Al2O3 and cleaned with ethyl alcohol and doubly distilled water in an ultrasonicator.
Apparatus and measurements
A CHI802a electrochemical analyzer (CH Instruments, Austin, TX, USA, http://chi.instrument.com.cn) was used to perform the voltammetric and electrochemical measurements. A PMT (model H9305-04, Hamamatsu Photonics K. K., Japan) with a spectral width of 185–830 nm was used to measure ECL emission. The ECL detections were carried out using a self-made glass cell and a three-electrode system consisted of a bare Au or a NPGL working electrode, a saturated calomel reference electrode and a Pt wire auxiliary electrode. During measurement, the working electrode was scanned from 0 to −2 V in 0.1 mol.L-1 phosphate buffered solution (pH 7.4) containing 0.1 mol.L-1 K2S2O8 and 0.1 mol.L-1 KNO3, and the ECL intensity (I ECL ) versus applied potential (E) was recorded.
Immunoassembly on NPGL electrode and QDs labeling
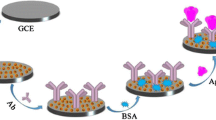
The NPGL electrode was firstly electrochemically pretreated in 0.5 mol.L-1 H2SO4 and washed with doubly distilled water. Then, the activated electrode was immersed in 0.01 mol.L-1 thioglycolic acid solution overnight. After being cleaned, the electrode was soaked in EDC (100 mg.mL-1 in H2O) and NHS (100 mg.mL-1 in H2O) for 1 h to activate the carboxy group on the surface of the electrode. Next, the electrode was incubated with 100 μL of 20 μg.mL-1 Ab1 at room temperature for 8 h and subsequently blocked with 20 μL of 2% BSA at 37 °C for 1 h in the constant-humidity chamber. Then, the electrode was incubated in 100 μL CEA for 3 h and in 100 μL of 2 μg.mL-1 Ab2 at 37 °C for 3 h. Finally, 40 μL of 1×10−4 mol.L-1 thioglycolic acid-capped CdTe QD solution containing 50 mg.mL-1 EDC and 50 mg.mL-1 NHS was dropped on the electrode and incubated at room temperature for 5 h. After each incubation, the electrode was washed five times with phosphate buffered solution (pH 7.4) and 1 mol.L-1 NaCl. The steps for immunoassembly and CdTe QDs labeling are illustrated in Scheme 1.
Results and discussion
Characterization of the NPGL electrode and ECL behaviors of CdTe QDs on NPGL electrode
NPGLs are large-area, crack-free, highly conductive and ultra-thin nanoporous gold membranes, which can be easily attached to a variety of substrates. Figure 1 displays the scanning electron microscopy (SEM) image of 100 nm thick NPGL with an uniform pore size distribution centered around 20–30 nm. Figure 2a shows the cyclic voltammetry of bare Au electrode (a) and NPGL electrode (b) in 0.5 mol.L-1 H2SO4. The result indicates a 6.9-fold enhancement for peak current on nanoporous gold leaf modified electrode compared with the bare Au electrode, it may because the NPGL electrode with more porous structure and larger surface area could facilitate the diffusion of H2SO4.
a Cyclic voltammograms of (a) bare Au electrode, (b) NPGL electrode in 0.5 mol.L-1 H2SO4; bI ECL -E curves of CdTe QDs labeled (a) bare Au electrode, (b) 100 nm thick NPGL electrode in 0.1 mol.L-1 phosphate buffered solution (pH 7.4) containing 0.1 mol.L-1 KNO3 and 0.1 mol.L-1 K2S2O8. Scan rate: 50 mV.s-1
The ECL of equivalent CdTe QDs on bare Au electrode (a), and NPGL electrode (b) were detected. The procedure of assembling CdTe QDs on to the electrode has been illustrated in Ref. [27]. In brief, the electrode was firstly immersed in cysteine solution. Then the compound of EDC, NHS and CdTe QDs were dropped onto the electrode which was then incubated in a constant humidity chamber. During the incubation, carboxyl group of CdTe QDs could covalently conjugate to the amino group of cysteine. As shown in Fig. 2b, the ECL intensity on NPGL electrode (curve b) was almost 5.5-fold higher than that obtained from the bare Au electrode (curve a). This could be explained that the NPGL electrode with porous structure and good conductivity can not only provide more binding sites for CdTe QDs assembling, but also facilitate the diffusion of K2S2O8 into the nanoporous.
Characterization of the immunoassembly and CdTe QDs labeling on NPGL electrode
The cyclic voltammogram of Fe(CN) 4-/3-6 is an effective method to probe the surface feature of electrode and monitor the barrier at modified electrode. When the electron transferred from solution species into electrode through tunnels of the barrier, it can be reflected on the redox peak current of the cyclic voltammogram. Thus, it was chosen as an indicator to investigate the changes of the electrode surface during each assembly step. Figure 3a shows the cyclic voltammograms of Fe(CN) 4-/3-6 at NPGL Au electrode with different modifiers, which records the surface feature of the electrode during the immunoassembly process. It can be seen that the peak current decreased after each assembly step, indicating the QDs labeled sandwich-typed immunocomplex were successfully immobilized on the electrode step by step. Each cyclic voltammogram was recorded after 10 cyclics to reach a steady-state response.
a Cyclic voltammograms of (a) NPGL electrode, (b) thioglycolic acid-modified NPGL electrode, (c) Ab2-Ag-Ab1-NPGL electrode and (d) QDs-Ab2-Ag-Ab1-NPGL electrode in 0.01 mol.L-1 Fe(CN) 4-/3-6 + 0.5 mol.L-1 KCl; bI ECL -E curves of (a) Ab2-Ag-Ab1-NPGL electrode, and CdTe QDs labeled Ab2-Ag-Ab1-NPGL electrode without (b) and with (c) EDC + NHS in 0.1 mol.L-1 phosphate buffered solution (pH 7.4) containing 0.1 mol.L-1 KNO3 and 0.1 mol.L-1 K2S2O8. Scan rate: 50 mV.s-1
Labeling CdTe QDs onto the immunocomplex at electrode was completed by covalent bonding between the carboxyl groups of CdTe QDs and the amine groups of biomolecules. Here, EDC [29] was chosen as the coupling agent with NHS as a stabilizing agent. Carboxylated CdTe QDs were firstly activated by EDC to form a stable active ester in the presence of NHS. Then, the active ester was reacted with the amine groups on the antibody, forming an amide bond between the CdTe QDs and proteins. As seen from Fig. 3b, after the immunoassembly of 5 ng.mL-1 CEA on the electrode, almost no ECL was observed before labeling CdTe QDs (curve a), implying that the background ECL signals of the system without CdTe QDs were very low. When the electrode was incubated in CdTe QDs without EDC and NHS, a weak ECL signal can be seen (curve b) resulting from the nonspecific adsorption of CdTe QDs on the electrode. However, when the electrode was incubated in CdTe QDs in the presence of EDC and NHS, a strong ECL signal was observed in curve c, indicating the CdTe QDs-Ab2 was successfully linked due to the addition of EDC and NHS and meanwhile the signal from adsorption can be negligible.
Detection of CEA
The immunoassay was carried out under the optimal detection conditions with different concentrations of CEA. As shown in Fig. 4a, the ECL intensity increased gradually with the increasing concentrations of CEA. This phenomenon implying that the ECL of CdTe QDs labeled on the electrode was corresponding to the concentration of CEA, which can be utilized for CEA determination consequently. The standard calibration curve for CEA detection is shown in Fig. 4b. The ECL intensity increases linearly with the increase of CEA concentrations in the range from 0.05 ng.mL-1 to 200 ng.mL-1 with a detection limit of 0.01 ng.mL-1 (R = 0.9973). According to the linear equation (logIECL = 0.269logCAg + 2.903), CEA concentration could be detected quantitatively.
aI ECL -E curves of CdTe QDs labeled immunocomplex on NPGL electrode in 0.1 mol.L-1 phosphate buffered solution (pH 7.4) containing 0.1 mol.L-1 KNO3 and 0.1 mol.L-1 K2S2O8. CEA concentration (ng.mL-1): (a) 0, (b) 0.05, (c) 0.5, (d) 5, (e) 10, (f) 50, (g) 100 and (h) 200. Scan rate: 50 mV.s-1; b Calibration curve of ECL intensity versus CEA concentration
The IECL-time curves of CdTe QDs labeled 200 ng.mL-1 CEA under continuous potential scaning from 0 to -2 V for 7 cycles is shown in Fig. 5. The coincident ECL signals are observed high and stable, indicating the good repeatability and stability of the ECL immunoassay for CEA at NPGL modified electrode.
Conclusions
In this work, we successfully applied the new ECL emitter quantum dots as lables for immunoassay with a new electrode material NPGL, which can effectively amplify the ECL intensity and thus improve the detection sensitivity. NPGL with the advantages of nanoporous structure and high conductivity would be a promising candidate for the development of new electrode materials. Besides, our successful application of QDs as labels in ECL immunoassay may extend a promising method for protein detection in clinical application.
References
Richter MM (2004) Electrochemiluminescence (ECL). Chem Rev 104:3003–3036
Miao WJ (2008) Electrogenerated chemiluminescence and its biorelated applications. Chem Rev 108:2506–2553
Wilson R, Clavering C, Hutchinson A (2003) Electrochemiluminescence enzyme immunoassays for TNT and pentaerythritol tetranitrate. Anal Chem 75:4244–4249
Eskola J, Mäkinen P, Oksa L, Loikas K, Nauma M, Jiang QH, Håkansson M, Suomi J, Kulmala S (2006) Competitive immunoassay by hot electron-induced electrochemiluminescence detection and using a semiautomatic electrochemiluminometer. J Luminescence 118:238–244
Wang SJ, Milam J, Ohlin AC, Rambaran VH, Clark E, Ward W, Seymour L, Casey WH, Holder AA, Miao WJ (2009) Electrochemical and electrogenerated chemiluminescent studies of a trinuclear complex, [((phen)2Ru(dpp))2RhCl2]5+, and its interactions with calf thymus DNA. Anal Chem 81:4068–4075
Zhan W, Bard AJ (2007) Electrogenerated chemiluminescence immunoassay of human C-reactive protein by using Ru(bpy) 2+3 -encapsulated liposomes as labels. Anal Chem 79:459–463
Tian DY, Duan CF, Wang W, Li N, Zhang H, Cui H, Lu YY (2009) Sandwich-type electrochemiluminescence immunosensor based on N-(aminobutyl)-N-ethylisoluminol labeling and gold nanoparticle amplification. Talanta 78:399–404
Bertoncello P, Forster RJ (2009) Nanostructured materials for electrochemiluminescence (ECL)-based detection methods: Recent advances and future perspectives. Biosens Bioelectron 24:3191–3200
Jie GF, Liu P, Wang L, Zhang SS (2010) Electrochemiluminescence immunosensor based on nanocomposite film of CdS quantum dots-carbon nanotubes combined with gold nanoparticles-chitosan. Electrochem Commun 12:22–26
Jie GF, Zhang JJ, Wang DC, Cheng C, Chen HY, Zhu JJ (2008) Electrochemiluminescence immunosensor based on CdSe nanocomposites. Anal Chem 80:4033–4039
Jie GF, Li LL, Chen C, Xuan J, Zhu JJ (2009) Enhanced electrochemiluminescence of CdSe quantum dots composited with CNTs and PDDA for sensitive immunoassay. Biosens Bioelectron 24:3352–3358
Jie GF, Liu B, Pan HC, Zhu JJ, Chen HY (2007) CdS nanocrystal-based electrochemiluminescence biosensor for the detection of low-density lipoprotein by increasing sensitivity with gold nanoparticle amplification. Anal Chem 79:5574–5581
Jie GF, Huang HP, Sun XL, Zhu JJ (2008) Electrochemiluminescence of CdSe quantum dots for immunosensing of human prealbumin. Biosens Bioelectron 23:1896–1899
Ding Y, Erlebacher J (2003) Nanoporous metals with controlled multimodal pore size distribution. J Am Chem Soc 125:7772–7773
Ding Y, Chen MW, Erlebacher J (2004) Metallic mesoporous nanocomposites for electrocatalysis. J Am Chem Soc 126:6876–6877
Ding Y, Kim YJ, Erlebacher J (2004) Nanoporous gold leaf: “ancient technology”/ advanced material. Adv Mater 16:1897–1900
Hammarström S (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9:67–81
Eppler E, Hörig H, Kaufman HL, Groscurth P, Filgueira L (2002) Carcinoembryonic antigen (CEA) presentation and specific T cell-priming by human dendritic cells transfected with CEA-mRNA. Eur J Cancer 38:184–193
Schneider J (2006) Tumor markers in detection of lung cancer. Adv Clin Chem 42:1
Gould DA, Moscoso GJ, Young MPA, Barton DP (2000) Human first trimester fetal ovaries express oncofetal antigens and steroid receptors. J Soc Gynecol Investig 7:131–138
Chu TM, Reynoso G (1972) Evaluation of a new radioimmunoassay method for carcinoembryonic antigen in plasma, with use of zirconyl phosphate gel. Clin Chem 18:918–922
Mekler VM, Bystryak SM (1992) Application of o-phenylenediamine as a fluorogenic substrate in peroxidase mediated enzyme-linked immunosorbent assay. Anal Chim Acta 264:359–363
Yuan JL, Wang GL, Majima K, Matsumoto K (2001) Synthesis of a terbium fluorescent chelate and its application to time-resolved fluoroimmunoassay. Anal Chem 73:1869–1876
Wang F, Hu SS (2009) Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta 165:1–22
Tang DY, Xia BY (2008) Electrochemical immunosensor and biochemical analysis for carcinoembryonic antigen in clinical diagnosis. Microchim Acta 163:41–48
Fu XH, Wang JY, Li N, Wang L, Pu L (2009) Label-free electrochemical immunoassay of carcinoembryonic antigen in human serum using magnetic nanorods as sensing probes. Microchim Acta 165:437–442
Hu XF, Wang RY, Ding Y, Zhang XL, Jin WR (2010) Electrochemiluminescence of CdTe quantum dots as labels at nanoporous gold leaf electrodes for ultrasensitive DNA analysis. Talanta 80:1737–1743
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Jiang KY, Schadle LS, Siegel RW, Zhang XJ, Zhang HF, Terrones M (2004) Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J Mate Chem 14:37–39
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 20975061), the National Basic Research Program of China (Grant No. 2007CB936602), the Natural Science Foundation of Shandong Province in China (Grant No. Y2008B20). We are grateful to Professor Yi Ding’s group (School of Chemistry and Chemical Engineering, Shandong University) for providing the NPGL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Wang, R. & Zhang, X. Electrochemiluminescence immunoassay at a nanoporous gold leaf electrode and using CdTe quantun dots as labels. Microchim Acta 172, 285–290 (2011). https://doi.org/10.1007/s00604-010-0487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0487-x