Abstract
A tyrosinase-based biosensor was constructed by immobilizing the enzyme on diazonium-functionalized screen-printed gold electrodes. Under optimized conditions, the biosensor exhibited rapid response to the changes in the concentration of all the tested phenolic compounds (catechol, catechin, caffeic acid and gallic acid). Sensitivity, linear range and limit of detection (LOD) were determined, and catechol was found to display the highest sensitivity (36.3 mA M−1) and the lowest LOD (0.1 μmol L−1). The biosensor was successfully applied to the detection of polyphenols in tea samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyphenolics are a broad group of compounds present in plant-derived foods including wine, tea, cacao or fruits [1]. Last years, some authors have associated the consumption of polyphenol-rich products with a reduction in the incidence of cardiovascular diseases, certain cancers and other diseases related to aging [2, 3]. This is related to the fact that their chemical structure enables polyphenols to act as antioxidant and anti-inflammatory compounds, scavenging and neutralizing free radicals, and inhibiting lipoprotein oxidation [4, 5].
The determination of both the content and the antioxidant activity of polyphenols in natural samples is extremely difficult. The tedious and complicated extraction processes, the complex polyphenols composition and the presence of interferences in the extracted samples impede a precise determination of the “total phenolic content”. Traditionally, the “total phenol content” has been determined by using spectrophotometric methods based on the reaction of phenolic compounds with a colorimetric reagent and the measurement of the developed color in the visible region [6]. However, these methods tend to overestimate the real content of phenolic compounds [7]. More accurate methods have been used nowadays, such as HPLC [8–10], capillary electrophoresis [11, 12] or chemiluminescence [13].These highly sensitive standard methods are expensive, time consuming and require formal training that considerably limits their wide spread use.
Amperometric biosensors are postulated as an attractive alternative for their rapid response, cost-effectiveness, simplicity of operation and manufacturing, minimal involved sample pretreatment and solvent requirements [14]. Most of biosensors currently used for the evaluation of the polyphenol content are based on polyphenol oxidases, tyrosinase [15–18] or laccase [19–21], or on peroxidase [22–24]. From all of them, tyrosinase biosensors are the most commonly applied to determine phenolic compounds in food samples. In the presence of oxygen, tyrosinase catalyses the oxidation of monophenols and o-diphenols to quinones. This enzymatic mechanism involved the emergence of two measurement approaches: (1) the amperometric detection of the oxygen consumption using a Clark-type electrode [17] and (2) the reversible electrochemical reduction of the o-quinone formed from phenols in the enzymatic reaction [25]. In both cases, the measured signal is proportional to the polyphenol concentration in solution. Classically, enzymatic immobilization protocols were based on either the physical entrapment or the covalent cross-linking of the enzyme with glutaraldehyde [17, 25, 26]. Last years, monolayer strategies have become increasingly popular because they provide controlled and oriented recognition interfaces [27, 28]. From early 1990s when Pinson and co-workers introduced the use of aryl diazonium salts for electrode modification [29], this immobilization scheme has been applied to a range of biosensing applications [30–37]. Advantages of this approach are a highly stable surface, ease of preparation and the ability to synthesize diazonium salts with a wide range of functional groups [38].
In this paper, a diazonium-functionalized tyrosinase-based biosensor for the detection of polyphenols in real samples of tea is presented. Screen-printed gold electrodes (SPGEs) were grafted with a p-nitrophenyl (p-NP) film by electrochemically reducing the in-situ generated p-nitrophenyl diazonium salt (p-NPDS), without isolation of the diazonium organic salt, followed by electroreduction of the nitro groups to amino groups. The amino-modified electrodes were further immobilized with tyrosinase using glutaraldehyde as a cross-linker, binding the enzyme amino groups to the electrode-surface amine. Diazonium-functionalized tyrosinase-based biosensors showed good response to phenolic compounds and were successfully applied to the detection of polyphenols in tea samples.
Experimental
Reagents
Tyrosinase from mushroom (EC 1.14.18.1, 5370 U per mg solid), catechol, catechin, caffeic acid, gallic acid, 4-nitroaniline, potassium chloride, sodium nitrite and Tween 20 were purchased from Sigma (www.sigmaaldrich.com). Hydrochloric acid and 25% glutaraldehyde were supplied from Prolabo (fr.vwr.com) and Aldrich (www.sigmaaldrich.com), respectively. All reagents were of analytical grade and were used without further purification. All solutions were prepared using Milli-Q water.
Preparation of the tea samples
Three different brands of black teas were purchased from a local market. Extraction of polyphenols was performed as follows: the tea bags were infused in 25 mL boiling water and kept at 90 °C for 5 min. The infusion was rapidly filtered through a Minisart syringe filter (pore size 5 μm; Sartorius, www.sartorius.com).
Equipment
All electrochemical measurements were performed with an Autolab PGSTAT 100 (Metrohm, www.metrohm.com). Cyclic voltammetry (CV) was employed to assess the electrochemical behavior of the modified electrodes whereas performance characteristics of the diazonium-functionalized tyrosinase-based biosensor were evaluated using chronoamperometry (CA). A three-electrode configuration was used for all the electrochemical measurements.
SPGEs, purchased from DropSens (www.dropsens.com), include three electrodes printed on a ceramic substrate (3.4 × 1.0 cm) and subjected to low-temperature curing. In terms of configuration, a gold disk-shaped (12.6 mm2) working electrode (WE) was surrounded by a gold counter electrode (CE) to ensure near-homogeneous polarisation of the WE [39]. A silver serigraphic electrode was included and used as pseudo-reference (pRE). All potential values were referred to the screen-printed silver pRE.
Biosensor fabrication
Prior to modification, electrodes were electrochemically cleaned by cycling the potential between 0 and +1.4 V at 100 mV s−1 in 0.1 mol L−1 H2SO4 until the characteristic cyclic voltammogram for a clean gold electrode was obtained.
The diazonium organic salt synthesis and the subsequent modification of the SPGE was performed as detailed in the literature [30]. The modification protocol scheme is represented in Fig. 1.
Briefly, the diazonium cations were synthesized in situ by reaction of 1.0 mmol L−1 sodium nitrite and 1.0 mmol L−1 4-nitroaniline in 0.5 mol L−1 HCl solution for 5 min (step 1). The diazonium salt was electrodeposited on the WE of SPGEs after one cycle from 0.4 to −0.4 V at 100 mV s−1 (step 2). SPGEs were then subjected to 5 potential scans between 0.4 and −1.25 V at 100 mV s−1 in 0.1 mol L−1 KCl for reduction of the p-NP film to p-aminophenyl (p-AP) (step 3). The amine on electrode surface was activated by incubation with 2.5% glutaraldehyde in 0.2 mol L−1 phosphate buffer pH 7.0 for 30 min (step 4). After washing with distilled water, 50 μL of tyrosinase solution (2.6 μg in 1.0 mL of 50 mmol L−1 phosphate buffer pH 7.0) were spread onto the WE surface and incubated for 60 min at room temperature (step 5). Finally, electrodes were washed with phosphate buffered solution + 0.1% Tween 20 (3 × 100 μL), 1 mol L−1 KCl (3 × 100 μL) and 0.1 mol L−1 phosphate buffer pH 7.0 (3 × 100 μL). Biosensors were kept dry at 4 °C until used.
Measurement protocol
Experiments were carried out in an electrochemical cell containing 5 mL of 0.1 mol L−1 phosphate buffer pH 7.0 by sequential addition of aliquots of either stock phenols solutions of known concentration or tea samples. After each addition, the sample was stirred for 30 s to ensure homogeneity before measuring. Chronoamperometric data were obtained by applying −200 mV for 120 s. Each calibration curve was derived from the average of three independent biosensors (n = 3).
Results and discussion
Evaluation of the SPGEs grafting via electrochemical reduction of diazonium salts
Diazonium-functionalized tyrosinase-based biosensors were prepared following the protocol described above. Figure 2a shows cyclic voltammograms recorded for the SPGEs in the diazotation mixture of 1 mmol L−1 NaNO2 and 1.0 mmol L−1 p-nitroaniline in 0.5 mol L−1 HCl (step 2, Fig. 1). An irreversible cathodic peak at −0.168 V/Ag pRE is observed. This peak was assigned to the reduction of diazonium species to aryl radicals, via one electron process. The aryl radicals coupled to the WE surface leading to irreversible grafting of a strongly bonded organic layer of p-NP. Very low currents were observed during the second and third voltammetric cycles, evidencing surface saturation and suggesting that a monolayer of fixed molecules was achieved.
a Cyclic voltammograms for in-situ-generated p-NPDS in the diazotation mixture (1.0 mmol L−1 NaNO2 +1.0 mmol L−1 4-nitroaniline in 0.5 mol L−1 HCl) at SPGEs; scan rate 100 mV s−1. b First (solid line) and last (dashed line) cyclic voltammograms of the p-NP-modified SPGE in 0.1 mol L−1 KCl; scan rate 100 mV s−1
Figure 2b plots the first and the last cyclic voltammogram of the p-NP to p-AP reduction process (step 3, Fig. 1). The voltammograms give rise to a split wave which corresponds to the irreversible reduction of grafted nitro groups in a multielectron and multiproton pathway, first to hydroxylamine derivatives (4e−/4H+) and further to the corresponding amines (2e−/2H+). The reduction of the wave indicates that nearly all the electroactive nitro groups are reduced.
The surface coverage of the attached p-NP was evaluated from the area under the reduction peak [40]. Indeed, a full surface coverage, Γ was attained from one cyclic voltammogram and a value of 6.90 × 10−10 mol cm−2 was found. This was consistent with the coverage found for the formation of close pack monolayer of grafted p-NP groups on glassy carbon electrode [41].
Optimization of the enzymatic biosensor: tyrosinase concentration and pH
In the optimization of the tyrosinase concentration, five biosensors were prepared following the protocol described in the experimental section by incubating each electrode with 2.0, 3.9, 6.9, 13.8 and 27.6 activity units of tyrosinase per mL (U mL−1), respectively. CA measurements were carried out in 15 μmol L−1 catechol. The current response of the sensor with the concentration of tyrosinase was plotted and it was found that the current response initially increased when increasing the concentration of tyrosinase until a maximum was reached at 13.8 U tyrosinase mL−1 (data not shown). Thus, from this point, all biosensors were prepared by incubating the WE with 13.8 U tyrosinase mL−1.
The enzyme activity is known to be seriously affected by the pH value of the buffer solution. The effect of the pH value was investigated in the range from 5.5 to 9.0 (Fig. 3). In these experiments, CA measurements were made using 15 μmol L−1 catechol in phosphate buffer at pH 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5 or 9. The maximum current response was obtained at the pH 7.0. Thus, in order to obtain the maximum sensitivity and bioactivity, a buffer of pH 7.0 was used throughout the research.
Repeatability, reproducibility and stability of the sensor
Repeatability, immobilization reproducibility and operational stability of the tyrosinase-based biosensors developed in the present work were evaluated.
In the repeatability analysis, biosensors were measured using CA in phosphate buffer containing 15 μmol L−1 catechol.
After 15 successive measurements, the repeatability of each electrode was analyzed in terms of the relative standard deviation (RSD). Tyrosinase-based biosensors showed good repeatability since in all cases the RSD never exceeded a magnitude of 5%.
The reproducibility was evaluated comparing the response of 5 biosensors prepared as previously detailed and measured using CA in phosphate buffer containing 15 μmol L−1 catechol. Reproducibility was again analyzed in terms of RSD and an accurate value of 5.8% was found.
The operational stability of the biosensor was analyzed with sensors prepared as before and successively measured during 8 h at 30 min intervals using CA in phosphate buffer containing 15 μmol L−1 catechol. Between measurements, the electrochemical cell was washed with buffer to eliminate catechol residues and biosensors were stored in buffer solution. After 15 successive substrate injections, a RSD of 8% was obtained. Long-term storage experiments showed high decreases in the sensor response. Considering the easy and rapid preparation protocol of the sensor (below 2 h), it is extremely recommended to prepare them every day.
Analysis of the response of the tyrosinase-based biosensor to phenolic compounds
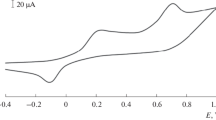
Sensors were prepared as previously detailed. The performance of the biosensor to phenolic compounds, precisely catechol, catechin, caffeic acid and gallic acid, was investigated by means of CA. Regarding Fig. 4a, the current response given by the electrochemical reduction of the o-quinone resulting from the enzyme reaction was detected at −200 mV/Ag pRE. Experimentally, 25, 25, 50, 50, 75, 75, 100, 100 and 100 μL of each phenolic compound (1 mmol L−1 in all cases) were sequentially introduced into 5 mL of phosphate buffer (pH 7) until no change was registered in the current response magnitude. After each addition, the solution was homogenized by stirring for 30 s before recording the current response. The CA data for catechol is plotted in Fig. 4b.
a Cyclic voltammogram of the o-quinone resulting of the action of the tyrosinase-based biosensors in 1 mmol L−1 catechol; scan rate 50 mV s−1. b CA data for consecutives additions of 15 μmol L−1 catechol by applying −200 mV/Ag pRE for 120 s. c Dependence of the CA reduction current on the concentration of catechol for the tyrosinase-based biosensor. Inset: Analytical performances of the tyrosinase-based biosensor. Average values were obtained with n = 3 biosensors
In each CA, the average of response intensities corresponding to the last 10 s of the measurement were taken as the intensity magnitude for the corresponding phenolic compound at that concentration. The representation of the obtained intensity magnitudes against the phenolic compound concentration was used as calibration curve (Fig. 4c, for catechol). The calibration curves of the phenolic compounds under study were used to evaluate the response of the tyrosinase-based biosensor to each molecule (Fig. 4, table inset). Calibration curves were analyzed in terms of sensitivity, linear range and limit of detection (LOD). The LOD was calculated according to the 3sb/m criterion, where sb and m are the standard deviation of the background current and the slope of the calibration plot, respectively. Huge differences in terms of sensitivity and LOD were observed when analyzing different phenolic substrates. Precisely, the sensitivity obtained with catechol was about 10 times bigger than the one obtained with the other compounds, which agrees with data reported by Zhang et al. [42], since catechol is the natural substrate of tyrosinase [43]. Moreover, the LOD of the biosensor to catechol is also the lowest and thus, catechol was taken as a reference and the “total phenol concentration” was always expressed as mg L−1 of equivalent catechol. It is worth to point out that in general very low LODs were obtained.
Characterization of the response of the biosensor to real samples of tea
The practical usefulness of the tyrosinase-based biosensor was evaluated by estimating the total phenolic content in teas. Three different brands of tea (samples designed as T1, T2 and T3) were purchased from a local supermarket. 100 μL of the tea decoction filtrate were injected to the electrochemical cell (final volume 5 mL) and CA measurements were performed. In the polyphenols determination, catechol was used as standard compound. The estimated concentration of total polyphenols in the tested teas were 0.77 ± 0.04 mg L−1, 1.34 ± 0.05 mg L−1 and 2.23 ± 0.07 mg L−1 for T1, T2 and T3, respectively, when considering catechol as a reference, and 18.8 ± 0.9 mg L−1, 32.4 ± 1.2 mg L−1 and 54 ± 2 mg L−1 for caffeic acid. These values, obtained for black teas, were in concordance with a previous publication of Ibarra-Escutia et al. [44], when taking into account the dilution factor regarding the different preparation protocol.
Conclusions
This paper describes for the first time the application of diazonium salts in the development of biosensors for the determination of the total phenol content and its application to real tea samples. The compact diazonium layer was advantageous since it should avoid the interference of negatively charged redox substances present in the tea matrix that may be oxidized or reduced at the applied potential. The enzymatic biosensor was constructed by immobilizing the tyrosinase enzyme onto diazonium functionalized screen-printed gold electrodes in a simple and fast protocol. The sensor response was optimized in terms of immobilized-enzyme concentration and buffer pH, obtaining best performances at 13.8 U tyrosinase mL−1 and pH 7. The biosensor exhibited good responses in terms of sensitivity, repeatability, reproducibility and operational stability to a number of phenolic compounds, especially to catechol, and was successfully applied to the detection of polyphenols in real samples of tea.
References
Valdés M, Valdés González A, García Calzón J, Díaz-García M (2009) Analytical nanotechnology for food analysis. Microchim Acta 166:1–19
Pratt DE (1992) Natural antioxidants from plant material. In phenolic compounds in food and their effects on health II. American Chemical Society, Washington, DC
Rice-Evans CA, Packer L (1989) Flavonoids in health and disease. CRC, Boca Raton, USA
Lu Y, Yeap Foo L (2000) Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem 68:81–85
Sánchez-Moreno C, Larrauri JA, Saura-Calixto F (1999) Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int 32:407–412
Blasco AJ, Rogerio MC, González MC, Escarpa A (2005) “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal Chim Acta 539:237–244
Escarpa A, González MC (2001) Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal Chim Acta 427:119–127
Cui H, He C, Zhao G (1999) Determination of polyphenols by high-performance liquid chromatography with inhibited chemiluminescence detection. J Chromatogr A 855:171–179
Ishii K, Furuta T, Kasuya Y (2001) Determination of rutin in human plasma by high-performance liquid chromatography utilizing solid-phase extraction and ultraviolet detection. J Chromatogr B 759:161–168
Ishii K, Furuta T, Kasuya Y (2003) High-performance liquid chromatographic determination of quercetin in human plasma and urine utilizing solid-phase extraction and ultraviolet detection. J Chromatogr B 794:49–56
Polásek M, Petriska I, Pospísilová M, Jahodár L (2006) Use of molybdate as novel complex-forming selector in the analysis of polyhydric phenols by capillary zone electrophoresis. Talanta 69:192–198
Xu X, Ye H, Wang W, Yu L, Chen G (2006) Determination of flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta 68:759–764
Song Z, Hou S (2002) Sensitive determination of sub-nanogram amounts of rutin by its inhibition on chemiluminescence with immobilized reagents. Talanta 57:59–67
Turner APF, Karube I, Wilson GS (1990) Biosensors: fundamentals and applications. Oxford University Press, USA
Montereali MR, Vastarella W, Della Seta L, Pilloton R (2005) Tyrosinase biosensor based on modified screen printed electrodes: measurements of total phenol content. Int J Environ An Ch 85:795–806
Romani A, Minunni M, Mulinacci N, Pinelli P, Vincieri FF, Del Carlo M, Mascini M (2000) Comparison among differential pulse voltammetry, amperometric biosensor, and HPLC/DAD analysis for polyphenol determination. J Agric Food Chem 48:1197–1203
Abhijith K, Sujith Kumar P, Kumar M, Thakur M (2007) Immobilised tyrosinase-based biosensor for the detection of tea polyphenols. Anal Bioanal Chem 389:2227–2234
Chen J, Jin Y (2010) Sensitive phenol determination based on co-modifying tyrosinase and palygorskite on glassy carbon electrode. Microchim Acta 169:249–254
Franzoi AC, Dupont J, Spinelli A, Vieira IC (2009) Biosensor based on laccase and an ionic liquid for determination of rosmarinic acid in plant extracts. Talanta 77:1322–1327
Gamella M, Campuzano S, Reviejo AJ, Pingarron JM (2006) Electrochemical estimation of the polyphenol index in wines using a laccase biosensor. J Agric Food Chem 54:7960–7967
Gomes SASS, Nogueira JMF, Rebelo MJF (2004) An amperometric biosensor for polyphenolic compounds in red wine. Biosens Bioelectron 20:1211–1216
S-i I, Kong Y-T, Watanabe M (2001) Amperometric biosensor for polyphenol based on horseradish peroxidase immobilized on gold electrodes. Electroanalysis 13:408–412
Mello LD, Alves AA, Macedo DV, Kubota LT (2005) Peroxidase-based biosensor as a tool for a fast evaluation of antioxidant capacity of tea. Food Chem 92:515–519
Kong Y-T, S-i I, Kano K, Ikeda T, Kakiuchi T (2001) Peroxidase-based amperometric sensor for the determination of total phenols using two-stage peroxidase reactions. Am J Enol Vitic 52:381–385
Carralero Sanz V, Mena ML, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2005) Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal Chim Acta 528:1–8
Eggins BR, Hickey C, Toft SA, Zhou DM (1997) Determination of flavanols in beers with tissue biosensors. Anal Chim Acta 347:281–288
Gooding JJ (2008) Advances in interfacial design for electrochemical biosensors and sensors: aryl diazonium salts for modifying carbon and metal electrodes. Electroanalysis 20:573–582
Griveau S, Mercier D, Vautrin-Ul C, Chaussé A (2007) Electrochemical grafting by reduction of 4-aminoethylbenzenediazonium salt: Application to the immobilization of (bio)molecules. Electrochem Commun 9:2768–2773
Bourdillon C, Delamar M, Demaille C, Hitmi R, Moiroux J, Pinson J (1992) Immobilization of glucose oxidase on a carbon surface derivatized by electrochemical reduction of diazonium salts. J Electroanal Chem 336:113–123
Radi A-E, Muñoz-Berbel X, Cortina-Puig M, Marty J-L (2009) An electrochemical immunosensor for ochratoxin A based on immobilization of antibodies on diazonium-functionalized gold electrode. Electrochim Acta 54:2180–2184
Radi A-E, Muñoz-Berbel X, Cortina-Puig M, Marty J-L (2009) Novel protocol for covalent immobilization of horseradish peroxidase on gold electrode surface. Electroanalysis 21:696–700
Radi A-E, Muñoz-Berbel X, Cortina-Puig M, Marty J-L (2009) A third-generation hydrogen peroxide biosensor based on horseradish peroxidase covalently immobilized on electrografted organic film on screen-printed carbon electrode. Electroanalysis 21:1624–1629
Yang X, Hall SB, Burrell AK, Officer DL (2001) A pH-responsive hydroquinone-functionalised glassy carbon electrode. Chem Commun 2628–2629
Vaik K, Mäeorg U, Maschion FC, Maia G, Schiffrin DJ, Tammeveski K (2005) Electrocatalytic oxygen reduction on glassy carbon grafted with anthraquinone by anodic oxidation of a carboxylate substituent. Electrochim Acta 50:5126–5131
Sarapuu A, Vaik K, Schiffrin DJ, Tammeveski K (2003) Electrochemical reduction of oxygen on anthraquinone-modified glassy carbon electrodes in alkaline solution. J Electroanal Chem 541:23–29
Nassef HM, Radi A-E, O’Sullivan CK (2006) Electrocatalytic sensing of NADH on a glassy carbon electrode modified with electrografted o-aminophenol film. Electrochem Commun 8:1719–1725
Zhou Y, Zhi J (2006) Development of an amperometric biosensor based on covalent immobilization of tyrosinase on a boron-doped diamond electrode. Electrochem Commun 8:1811–1816
Pinson J, Podvorica F (2005) Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem Soc Rev 34:429–439
Harrar JE, Shain I (1966) Electrode potential gradients and cell design in controlled potential electrolysis experiments. Anal Chem 38:1148–1158
Laviron E (1979) The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J Electroanal Chem 100:263–270
Liu Y-C, McCreery RL (1995) Reactions of organic monolayers on carbon surfaces observed with unenhanced raman spectroscopy. J Am Chem Soc 117:11254–11259
Zhang X, van Leeuwen J, Wichers HJ, Flurkey WH (1999) Characterization of tyrosinase from the cap flesh of portabella mushrooms. J Agric Food Chem 47:374–378
Seo S-Y, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51:2837–2853
Ibarra-Escutia P, Gómez JJ, Calas-Blanchard C, Marty JL, Ramírez-Silva MT (2010) Amperometric biosensor based on a high resolution photopolymer deposited onto a screen-printed electrode for phenolic compounds monitoring in tea infusions. Talanta 81:1636–1642
Acknowledgments
The authors greatly acknowledge the European Commission for financial support through the project “Nutra-Snacks” (FOOD-CT-2005-023044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cortina-Puig, M., Muñoz-Berbel, X., Calas-Blanchard, C. et al. Diazonium-functionalized tyrosinase-based biosensor for the detection of tea polyphenols. Microchim Acta 171, 187–193 (2010). https://doi.org/10.1007/s00604-010-0425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0425-y