Abstract
Ultrasound-assisted emulsification microextraction was applied to extract the herbicides simazine, atrazine, prometon, ametryn and prometryn from soil samples. They then were determined by HPLC with diode-array detection. Parameters that affect the extraction efficiency, such as the kind and volume of the extraction solvent, emulsification time and addition of salt, were optimized. Under the optimum conditions, the following analytical figures of merits are found: enrichment factors between 145 and 222, limits of detection between 0.1 to 0.5 ng g−1, analytical linearity in the range from 1.0 to 200 ng g−1, correlation coefficients (r) between 0.9989 and 0.9998, relative standard deviations from 2.8% to 3.6% (at n = 5, intraday) and 3.7% to 4.3% (interday), and recoveries (at spiking levels of 5.0 and 50.0 ng g−1) from 82.6% to 92.0%. The technique is simple, practical, rapid, and environmentally friendly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazine herbicides have been extensively used for weed control in agriculture in the last three decades. Triazines and their degradation products are very toxic, highly resistant, and can survive many years in the soil, water and organisms. Therefore, they are considered one of the most important classes of chemical pollutants. Moreover, atrazine has been classified as human carcinogen [1]. So, sensitive and selective analytical methods are desirable for the determination of triazine herbicide residues in different sample matrices.
Different analytical methods, such as gas chromatography-mass spectrometry (GC–MS) [2–4], high-performance liquid chromatography (HPLC) [5–7] and capillary electrophoresis [8], have been developed for the separation and quantification of triazine herbicides. To achieve the necessary levels of sensitivity, an enrichment step is usually needed before the chromatographic analysis. For the determination of triazine herbicides, different sample preparation methods have been developed, including liquid–liquid extraction (LLE) [9], solid-phase extraction (SPE) [10], solid-phase microextraction (SPME) [4, 6], supercritical fluid extraction (SFE) [11], microwave-assisted solvent extraction (MASE) [7], and molecularly imprinted SPE [12, 13].
Recently, much attention is being paid to the development of miniaturized, more efficient and environmentally friendly extraction techniques that could greatly reduce the organic solvent consumptions [14]. For this purpose, several different types of LPME techniques, such as single-drop microextraction (SDME) [2, 15] and dispersive liquid–liquid microextraction (DLLME) [3, 5], have been applied for the extraction of triazine herbicides in different samples. LPMEs have advantages of simplicity, effectiveness, low cost, and minimum use of solvents. They can also overcome some disadvantages often encountered in SPME, such as sample carry-over, the requirement to condition the SPME fiber, and additional instrumental modification. However, several disadvantages also exist in LPMEs. For example, the instability of liquid drop and long analysis time are often encountered in SDME; in DLLME, the main drawbacks are the difficulty to automation and the requirement to use an organic dispersive solvent to enhance the dispersion of the extraction solvent in the aqueous phase, which usually decreases the partition coefficient of analytes into the extraction solvent.
More recently, a novel microextraction technique, named ultrasound-assisted emulsification microextraction (USAEME), has been developed by Garcia-Jares and co-workers [16], which is based on the emulsification of a microvolume of water-immiscible extraction solvent in the sample aqueous solution by ultrasound radiation. The application of ultrasonic radiation accelerates the mass-transfer process of the analytes between the two immiscible phases, which, together with the large surface of contact between the two phases, leads to an increment in the extraction efficiency in a minimum amount of time. The analytes in the sample are extracted into the fine droplets. The two phases can be readily separated by centrifugation and the enriched analytes in the sedimented phase can be determined by either chromatographic or spectrometric methods. The advantages of the combination of the micro-extracting systems with ultrasounds radiation include high efficiency, low cost, low organic solvent consumption and simplicity of operation. USAEME has been used for the determination of synthetic musk fragrances, phthalate esters and lindane [16], polybrominated diphenyl ethers [17], polycyclic aromatic hydrocarbons [18], phenolic preservatives [19], polychlorinated biphenyls [20], organochlorine herbicides [21] and propoxur [22] in water and beverage samples.
In continuation to our previous explorations of novel sample pretreatment techniques [23–27], now, a USAEME method coupled with HPLC-diode array detection (DAD) has been developed for the determination of triazine herbicides in soil samples. The effects of various experimental parameters, such as the kind and volume of the extraction solvent, ultrasound emulsification time and salt addition, were investigated and optimized. The method is simple, practical, rapid and environmentally friendly.
Experimental
Reagents and materials
Simazine, atrazine, prometon, ametryn and prometryn were purchased from Agricultural Environmental Protection Institution of Tianjin (Tianjin, China). Chloroform (CHCl3), tetrachloride ethylene (C2Cl4), carbon tetrachloride (CCl4), chlorobenzene, were purchased from Beijing Chemical Reagents Company (Beijing, China). Methanol was from Sinopharm Chemical Reagent Co. (Beijing, China). Sodium chloride (NaCl) was from Tianjin Fuchen Chemical Reagent Factory (Tianjin, China). The water used throughout the work was double-distilled on a SZ-93 automatic double-distiller purchased from Shanghai Yarong Biochemistry Instrumental Factory (Shanghai, China).
Soil samples which were representative of agricultural soils in this local area were collected from the plough layer of the mealie field at Biaobenyuan and Wumazhuang (Baoding, China). They were dried at room temperature, pulverized and passed through 250-μm sieve. All the solvents and soil sample extraction solutions were filtered through a 0.45-μm membrane to eliminate particulate matters before analysis.
A mixture stock solution containing simazine, atrazine, prometon, ametryn and prometryn at 10.0 μg mL−1 was prepared in methanol. A series of standard solutions were prepared by mixing an appropriate amount of the stock solution with double-distilled water in a 10-mL volumetric flask. All the standard solutions were stored at 4 °C in the dark.
Instruments
The HPLC system, assembled from modular components (Waters, http://www.waters.com), consisted of an in-line degasser, a 600E pump, and a DAD detector. A Millennium workstation (Waters, http://www.waters.com) was utilized to control the system and for the acquisition and analysis of the data. A Centurysil C18 column (4.6 i.d. × 250 mm, 5.0 μm) from Dalian Jiangshen Separation Science Company (Dalian, China) was used for separations. The mobile phase was a mixture of methanol-water (60:40 v/v) at a flow rate of 1 mL min−1. The DAD monitoring wavelengths were chosen at 222 nm for the six triazine herbicides.
Extractions were performed at 40 kHz of ultrasound frequency and 80 W of power on a KQ-2200 DE ultrasonic water bath purchased from Kunshan Ultrasonic Instruments Co. Ltd. (http://www.ks-csyq.com).
USAEME procedure
Soil samples were air-dried at room temperature, pulverized and passed through 250-μm sieve. 10.0 g of the soil sample was accurately weighed and put into a 50 mL centrifuge tube, to which 10.0 mL double-distilled water was added. The resultant sample mixture was first vigorously shaken on a vibrator for 40 min and then filtrated under reduced pressure. The filtrate was transferred to a 10 ml volumetric flask, to which, water was added to complete the volume. For the USAEME, a 5.0 mL aliquot of the above sample solution was placed in a 10 mL screw cap glass tube with conical bottom. 100 μL of C6H5Cl was added into the sample solution. The resulting mixture was then immersed into an ultrasonic bath at 25 ± 2 °C for 3 min of sonication. The emulsion was disrupted by centrifugation at 3,500 rpm for 5 min and the organic phase was sedimented at the bottom of the centrifuge tube. The sedimented phase was completely transferred to another test tube with conical bottom using 100-μL HPLC syringe and then evaporated to dryness under a mild nitrogen stream. The residue was dissolved in 20.0 μL methanol and 15.0 μL was injected into the HPLC system for analysis.
Calculation of enrichment factor and extraction recovery
In order to evaluate the effect of different experimental parameters such as the type and volume of the extraction and disperser solvents, salt addition, and the extraction time on the performance of DLLME, the terms of the enrichment factor (EF) and the extraction recovery (R) were introduced and used according to the Eqs. 1 and 2 as follows [24, 25]:
where EF, C inj and C 0 are the enrichment factor, the analyte concentration in the injection solution and the initial analyte concentration in the aqueous samples, respectively.
where R%, 0.02 and V aq are the extraction recovery, the volume of the reconstituted solution (mL) and the volume of the aqueous sample (mL), respectively.
Results and discussion
Water was used as the extraction solvent for the extraction of the triazines from soil, which was adapted from Ref. [4]. For the optimization of USAEME, 5.0 mL of double-distilled water spiked with 50.0 ng g−1 each of the five triazine herbicides was used to study the extraction performance under different experimental conditions. All the experiments were performed in triplicate and the means of the results were used for optimization. The influence of the main variables that potentially affect the efficiency of the USAEME, was evaluated.
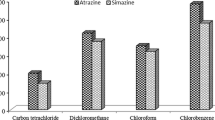
Selection of extraction solvent
The selection of an appropriate extraction solvent is critical to the UASEME process since its physicochemical properties not only affect the emulsification phenomenon but also the extraction efficiency. The extraction solvent should meet the following requirements: it should have a higher density than water, a low solubility in water, high extraction capability for the target analytes, and form a stable emulsion system with the help of ultrasound radiation. Based on these considerations, CCl4, CHCl3, C2Cl4 and C6H5Cl were selected as potential extraction solvents for the study. Figure 1 shows the effect of the extraction solvents on the enrichment factor. As can be seen in Fig. 1, among the four solvents investigated, C6H5Cl gives the highest extraction efficiency. Therefore C6H5Cl was selected as the extraction solvent.
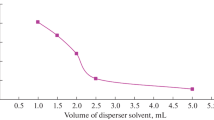
Effect of extraction solvent volume
In order to study the effect of the volume of the extraction solvent on the performance of the presented USAEME procedure, the volume of C6H5Cl was varied in the range from 50 to 200 μL. When the volume of the extraction solvent was increased, the enrichment factor were increased until 100 μL. At higher volumes than 100 μL, the enrichment factor almost remained constant or slightly decreased (Fig. 2). From the obtained results, 100 μL of C6H5Cl was chosen for further studies.
Effect of sample solution pH
The pH of sample solution is another important factor that affects the extraction performance. The effect of sample pH in the range of 5.0–12.0 on the extraction of the triazine herbicides was investigated. The results indicated that the extraction efficiency remained almost constant at pH 6.0–9.0 and was higher than that at pH < 6.0 and pH > 9.0. Maybe this was because the triazines were easily degraded in either strong acidic or alkali conditions. The pH of the aqueous sample solution was about 6.3, so its pH was not adjusted.
Effect of extraction temperature
Temperature could affect both mass-transfer and emulsification process, thus influencing the extraction efficiency. The effect of extraction temperature was studied over the temperatures ranging from 20 °C to 40 °C. In the whole temperature range from 20 °C to 40 °C, the emulsification was all easily achieved and maintained well in the whole extraction time. The results indicated that the sample solution temperature had no significant effect on the extraction recoveries of the triazines. This may be due to that the contact surface between the organic solvent and the aqueous phase is very large and mass transfer is not a limiting factor for the USAEME. Therefore, the extractions were carried out at room temperature (25 ± 2 °C) for the convenience.
Effect of ultrasound extraction time
Extraction time is usually an important factor in the most of extraction procedures. Ultrasound extraction time is one of the main factors in USAEME as in most extraction procedures. It affects both emulsification and mass transfer process, and thus influences the extraction recovery of the analytes. The sonication extraction time was defined as the time interval between the addition of the extraction solvent (C6H5Cl) to the sample to start the sonication and the end of the sonication stage. The effect of the sonication extraction time was studied over 1 to 9 min. The results (Fig. 3) indicated that the enrichment factors are increased by increasing the extraction time before 3 min, and after that, remained almost constant or gradually decreased. Therefore, 3 min of the sonication time was chosen for the experiments.
Effect of salt addition
To evaluate the possibility of salting out effect, the enrichment factor was studied over the NaCl concentration range from 0 to 13% (w/v) while the other parameters were kept constant. The results indicated that there was a decrease in enrichment factor with the increase of the salt concentration. Although the addition of salt could decrease the solubility of analytes in the aqueous phase and promote the transfer of the analytes towards the organic phase, however, it could also increase the viscosity of the solution. The viscosity of sample solution plays an important role in the USAEME since ultrasounds could be absorbed by the viscous resistance of the solution and dispersed as calorific energy. As a consequence, the organic phase was not able to be dispersed in so fine droplets and therefore, the efficiency of emulsion formation could be drastically reduced and the extraction efficiency decreased with the addition of NaCl. Hence, NaCl was not added in all the subsequent experiments.
As a result, under the optimum conditions, the enrichment factors were in the range between 145 and 222.
Evaluation of the method
Linearity, repeatability and limits of detection (LODs)
For the establishment of the calibration curve, the soil samples, which were free of the analytes, were spiked with each of simazine, atrazine, prometon, ametryn and prometryn at six concentration levels of 1.0, 5.0, 10.0, 20.0, 50.0, 100.0 and 200.0 ng g−1, respectively. For each level, five replicate extractions were performed. Linearity was observed in the range from 1.0 to 200.0 ng g−1 with the correlation coefficients (r) ranging from 0.9989 to 0.9998. The repeatability study was evaluated in terms of intraday and interday precisions, by extracting and analyzing the spiked soil samples at the concentration of each herbicide at 10 ng g−1 in the same day and on the three consecutive days. The resultant intraday and interday repeatabilities expressed as relative standard deviations (RSDs) varied from 2.8% to 3.6% and 3.7% to 4.3%. The LODs for soil samples, based on a signal-to-noise ratio (S/N) of 3, was 0.5 ng g−1 for simazine, and 0.1 ng g−1 for atrazine, prometon, ametryn and prometryn, respectively. The above calibration data are listed in Table 1. These results show that the proposed method has a high sensitivity and repeatability.
Soil samples analysis and recoveries of the method
To evaluate the applicability of the proposed method, the extraction and determination of the five triazines in different soil samples were performed. As a result, no residues of the target triazines were found in the Biaobenyuan soil sample. For Wumazhuang soil sample, atrazine was found to be at 3.1 ng g−1.
To test the accuracy of the method, these soil samples were spiked with the standards of the target analytes at the concentrations of 5.0 and 50.0 ng g−1, respectively. For each concentration level, five replicate experiments for a whole analysis process as described in the experimental section were made. The recoveries of the method were expressed as the mean percentage between the amounts found and the ones added. The results are given in Table 2. The recoveries for the triazines in soil samples were in the range from 82.6% to 92.0%. Figure 4A and B showed the typical chromatograms of the extracted triazines from Wumazhuang soil sample before and after being spiked at 5 ng g−1 each of the five triazines.
Conclusions
In this paper, a novel ultrasound-assisted emulsification microextraction technique coupled with HPLC-DAD detection has been developed for the determination of triazines in soil samples. Compared with other conventional sample preparation methods, such as LLE and SPE, the analytical technique offers advantages such as simplicity, ease of operation, relatively short analysis time, and lower consumption of organic solvent. The results indicate that the method can provide a good repeatability, high enrichment factor and good recovery with a short analysis time, and can be used as an alternative of choice for the simple and efficient extraction and preconcentration of the compounds in soil samples.
References
Jiang H, Adams C, Graziano N, Roberson A, McGuire M, Khiari D (2006) Occurrence and removal of chloro-s-triazines in water treatment plants. Environ Sci Technol 40:3609
Bagheri H, Khalilian F (2005) Immersed solvent microextraction and gas chromatography–mass spectrometric detection of s-triazine herbicides in aquatic media. Anal Chim Acta 537:81
Nagaraju D, Huang SD (2007) Determination of triazine herbicides in aqueous samples by dispersive liquid–liquid microextraction with gas chromatography–ion trap mass spectrometry. J Chromatogr A 1161:89
Zambonin CG, Palmisano F (2000) Determination of triazines in soil leachates by solid-phase microextraction coupled to gas chromatography–mass spectrometry. J Chromatogr A 874:247
Zhou QX, Pang L, Xie GH, Xiao JP, Bai HH (2009) Determination of Atrazine and simazine in environmental water samples by dispersive liquid–liquid microextraction with high performance liquid chromatography. Anal Sci 25:73
Huang SD, Huang HI, Sung YH (2004) Analysis of triazine in water samples by solid-phase microextraction coupled with high-performance liquid chromatography. Talanta 64:887
Cheng JH, Liu M, Zhang XY, Ding L, Yu Y, Wang XP, Jin HY, Zhang HQ (2007) Determination of triazine herbicides in sheep liver by microwave-assisted extraction and high performance liquid chromatography. Anal Chim Acta 590:34
Carabias-Martinez R, Rodriguez-Gonzalo E, Miranda-Cruz E, Dominguez-Alvarez J, Hernandez-Mendez J (2006) Comparison of a non-aqueous capillary electrophoresis method with high performance liquid chromatography for the determination of herbicides and metabolites in water samples. J Chromatogr A 1122:194
Sabik H, Jednnot R (1998) Determination of organonitrogen pesticides in large volumes of surface water by liquid–liquid and solid-phase extraction using gas chromatography with nitrogen–phosphorus detection and liquid chromatography with atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 818:197
Do Nascimento PC, Rohlfes ALB, Bohrer D, De Carvalho LM, Pilau EJ (2005) HPLC based method using sample precolumn cleanup for the determination of triazines and thioltriazines in hemodialysis saline solutions. Talanta 65:211
Knez Z, Rizner-Hras A, Kokot K, Bauman D (1998) Solubility of some solid triazine herbicides in supercritical carbon dioxide. Fluid Phase Equilibria 152:95
Cacho C, Turiel E, Martin-Esteban A, Ayala D, Perez-Conde C (2006) Semi-covalent imprinted polymer using propazine methacrylate as template molecule for the clean-up of triazines in soil and vegetable samples. J Chromatogr A 1114:255
Hu XG, Hu YL, Li GK (2007) Development of novel molecularly imprinted solid-phase microextraction fiber and its application for the determination of triazines in complicated samples coupled with high-performance liquid chromatography. J Chromatogr A 1147:1
Sarafraz-Yazdi A, Amiri A (2009) Liquid-phase microextraction. Trends Anal Chem 29:1
Ye CL, Zhou QX, Wang XM (2007) Improved single-drop microextraction for high sensitive analysis. J Chromatogr A 1139:7
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R (2008) Ultrasound-assisted emulsification–microextraction of emergent contaminants and pesticides in environmental waters. J Chromatogr A 1190:27
Fontana AR, Wuilloud RG, Martinez LD, Altamirano JC (2009) Simple approach based on ultrasound-assisted emulsification-microextraction for determination of polyibrominated flame retardants in water samples by gas chromatography–mass spectrometry. J Chromatogr A 1216:147
Saleh A, Yamini Y, Faraji M, Rezaee M, Ghambarian M (2009) Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6673
Regueiro J, Llompart M, Psillakis E, Garcia-Monteagudo JC, Garcia-Jares C (2009) Ultrasound-assisted emulsification–microextraction of phenolic preservatives in water. Talanta 79:1387
Ozcan S, Tor A, Aydin ME (2009) Determination of selected polychlorinated biphenyls in water samples by ultrasound-assisted emulsification–microextraction and gas chromatography–mass-selective detection. Anal Chim Acta 647:182
Ozcan S, Tor A, Aydin ME (2009) Application of ultrasound-assisted emulsification–microextraction for the analysis of organochlorine pesticides in waters. Water Res 43:4269
Wu JF, Xiang BR, Xia J (2009) Application of ultrasound-assisted emulsification–microextraction combined with high performance liquid chromatography to the determination of propoxur in environmental and beverage samples. Microchim Acta 166:157
Wu QH, Wang C, Liu ZM, Wu CX, Wang Z (2009) Dispersive solid-phase clean-up followed by dispersive liquid–liquid microextraction for the determination of sulfonylurea herbicides in soil by high performance liquid chromatography. J Chromatogr A 1216:5504
Wu QH, Li YP, Wang C, Liu ZM, Zang XH, Xin Z, Wang Z (2009) Dispersive liquid–liquid microextraction combined with high performance liquid chromatography–fluorescence detection for the determination of carbendazim and thiabendazole in environmental samples. Anal Chim Acta 638:139
Wu QH, Xin Z, Li YM, Zang XH, Wang C, Wang Z (2009) Application of dispersive liquid–liquid microextraction combined with high performance liquid chromatography to the determination of carbamate pesticides in real water samples. Anal Bioanal Chem 393:1755
Zang XH, Wang JT, Wang O, Wang MZ, Ma JJ, Xi GH, Wang Z (2008) Analysis of captan, folpet and captafol in apples by dispersive liquid–liquid microextraction combined with gas chromatography. Anal Bioanal Chem 392:749
Wang C, Li CR, Zang XH, Han DD, Liu ZM, Wang Z (2007) Hollow fiber-based liquid-phase microextraction combined with on-line sweeping for trace analysis of Strychnos alkaloids in urine by micellar electrokinetic chromatography. J Chromatogr A 1143:270
Acknowledgments
Financial support from both the Natural Science Foundations of Hebei (B2008000210 and B2010000657) and the Scientific Research Foundation of Education Department of Hebei Province (2009132) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Li, Z., Wu, C. et al. Application of ultrasound-assisted emulsification microextraction for the determination of triazine herbicides in soil samples by high performance liquid chromatography. Microchim Acta 170, 59–65 (2010). https://doi.org/10.1007/s00604-010-0385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0385-2