Abstract
A DNA-modified carbon paste electrode (DNA-CPIE) was designed by using a mixture of the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate and paraffin oil as the binder. The electrochemistry of rutin at the DNA-CPIE was investigated by cyclic voltammetry and differential pulse voltammetry. Rutin exhibits a pair of reversible redox peaks in buffer solutions of pH 3.0, and respective electrochemical parameters are established. Under the optimal conditions, the oxidative peak current is linear with the concentration of rutin in the range from 8 × 10−9 to 1 × 10−5 mol L−1, and the detection limit is 1.3 × 10−9 mol L−1 (at S/N = 3). The electrode exhibits higher sensitivity compared to DNA modified carbon paste electrode without ionic liquid and better selectivity comparing with electrodes without DNA. It also showed good performance, stability, and therefore represents a viable method for the determination of rutin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are kinds of ionic compounds, which exist in liquid state at around room temperature. Because of their high electrochemical stability, good ionic conductivity, negligible vapor pressure and wide electrochemical windows [1], ILs have received much attention in the electrochemistry industry [2, 3], organic synthesis [4], catalysis [5], liquid-liquid extraction [6], ect. Recently, ILs have been used as the paste binders for fabricating of carbon composite electrodes, which provide an obvious increase in the electrochemical response of electroactive substrates and reduce the overpotentials of some organic substances [7]. Makeli et al. [8] fabricated a new carbon composite electrode using carbon powder mixed with IL as the binder, which was used for investigating the electrochemical oxidation of phenolic compounds. High stable responses were obtained even at high concentrations of phenol. Sun et al. [9] investigated the electrochemical behaviors of ascorbic acid (AA) at the IL modified carbon paste electrode, which decreased the overpotential of AA. These excellent results indicate that using IL-type carbon paste electrode for the determination of small molecules is promising.
DNA is one of the most important biomacromolecules, which offers the electrochemists a powerful tool to obtain necessary information for the development of various fields. For example, DNA recognition layers offer enormous promise in monitoring of many important compounds [10]. They are rapidly developed in the detection of nucleotide sequences, DNA damage, and genetic diseases [11]. This technique is also applied in drug monitoring [12] and environmental research [13] for its low cost and rapid response. Due to the high charge density of the DNA helix and the binding ability for various molecules, DNA is an available electrodes modification material with good selectivity and sensitivity.
Rutin is called as vitamin P that belongs to flavonoid compounds. Its several physiological activities including analgesic [14] and anticancer activities [15] have been widely exploited due to its biological effects [16]. There has been considerable effort in the development of analytical methods for the determination of rutin including capillary electrophoresis [17], high-performance liquid chromatography [18], chemiluminescence [19] and sequential injection analysis [20]. Some of the methods require large amounts of toxic organic solvents or complex isolation processes. Thereby, electrochemical methods have attracted extensive interests because of its high sensitivity, simple preparation and low costs. The previous electrochemical researches about rutin were mainly performed on single-walled carbon nanotube modified gold electrode (SWNT-Au) [21] and single-sided heated graphite cylindrical electrodes [22]. In this paper, A DNA modified carbon paste electrode (DNA-CPIE) based on the mixture of IL and paraffin oil as the binder for rutin monitoring was reported. The electrochemical behaviors of rutin at the DNA-CPIE were investigated and a sensitive voltammetric method for rutin determination was developed.
Experimental
Reagents and materials
DNA was obtained from Biosharp and was prepared with phosphate-buffered saline (PBS) (0.05 mol L−1, pH 7.0). Rutin was purchased from Shanghai Boyun Biotech Co. Ltd (Shanghai, China, www.chem-china.net). Its stock solution (2 × 10−3 mol L−1) was prepared by dissolving it with ethanol. Carbon powder (spectrum grade, average particle size 4 μm) and paraffin oil were from Shanghai Chemical Reagent Co (Shanghai, China, www.scrri.com). Rutin tablet was purchased from Taiyuan Pharmaceutical Co. Ltd (Taiyuan, China, www.sxtaiyao.com.cn). The supporting electrolyte was 0.1 mol L−1 Britton-Robinson (B-R, pH 3.0). All other chemicals were of analytical reagent. All solutions were prepared with double-distilled water.
1-Butyl-3-methylimidazolium hexafluorophosphate ([BMIM] [PF6]) was prepared as described in the literatures [23, 24]. The purity of IL was checked by elemental analysis and 1H NMR and 13C NMR spectroscopy, and the residual water content was analyzed by standard Karl-Fisher titration to be below 0.07% (w/w).
Preparation of the modified electrode
The conventional carbon paste electrode (CPE) was constructed by mixing carbon powder with paraffin oil in weight ratio of 85:15. The CPIE was prepared by adding [BMIM] [PF6] to the conventional carbon paste, and the optimal paste was comprised of carbon powder, paraffin oil, and IL for 80:15:5. Mixing proceeded for an additional 30 min to form a homogeneous paste. A portion of the paste was then packed tightly into the cavity (2 mm diameter, 2 mm depth) of a Teflon tube and a bare copper wire had been inserted through the opposite end to produce electrical contact. The composite surface was smoothed on a weighing paper and rinsed carefully with double-distilled water. The obtained composites were uniform and the surfaces of the prepared electrodes were smooth, indicating that there was good conglutination to IL with carbon powder and paraffin oil. The DNA-CPIE was designed by transferring a droplet of 30 μL of 1 mg mL−1 DNA solution onto the composite surface, followed by air-drying overnight.
Apparatus and procedures
All electrochemical experiments were performed on a CHI 660 electrochemical workstation (CH Inc., USA, www.chinstruments.com). A standard three-electrode system was used with the DNA-CPIE as a working electrode, a platinum wire as an auxiliary electrode, and a saturated calomel electrode (SCE) as a reference electrode. All potentials given were referred to the SCE. The microstructure characterization of carbon paste electrode surfaces was performed with the Navo 400 Scanning Electron Microscope (SEM, FEI Company of USA). All measurements were performed at ambient temperature.
Results and discussion
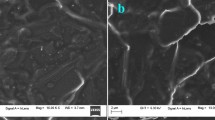
The surface topography of the paste electrodes
The morphologies of CPE and CPIE were characterized by SEM. The surface of CPE was predominated by isolated and brokenly shaped graphite flakes, and each layer could be distinctly distinguished (Fig. 1a). After adding the IL to the paste, the surface of CPIE was relatively uniform and few separated layers could be seen (Fig. 1b). The uniformity of surface showed the good adherence of carbon powder, paraffin oil and IL. A mass of IL was embedded in carbon powder and paraffin oil, which bridged carbon flakes [25].
Electrochemical behaviors of rutin
The electrochemical behaviors of rutin were studied by cyclic voltammetry (CV). In 1.0 × 10−4 mol L−1 rutin, a couple of well-defined peaks could be seen at the DNA-CPIE with oxidative peak potential (E pa) of 0.488 V, reductive peak potential (E pc) of 0.427 V (Fig. 2c). The peak-peak separation (∆E p) was 61 mV. The peak couple attributed to the redox of dihydroxyl groups at B-ring [26]. It was obvious that the current at the DNA-CPIE was higher than that at the DNA-CPE (Fig. 2a). The higher current might be due to the use of IL as binder, which resulted in an increase in the electron transfer rate [27]. On the one hand, IL had high ionic conductivity that could facilitate fast transportation. It would induce the reduction of surface diffusion capacitance. On the other hand, there were many cavities within its molecule structure that was easy to hold more charges. Thus, IL as a binder could enhance electrochemical performance of the DNA-CPIE [28]. The peak current of rutin at the DNA-CPIE decreased comparing with at the CPIE (Fig. 2b). It indicated that DNA and rutin had a strong interaction and formed weakly electroactive rutin-DNA complex, which resulted in the decrease of peak current [26]. A pair of peaks at the DNA-CPIE could be observed after the addition of 4.63 × 10−6 mol L−1 rutin into B-R (pH 3.0) buffer solution (Fig. s1a, see Supplementary material). Then the electrode was removed from the solution, thoroughly rinsed with pure water and put into B-R (pH 3.0) buffer solution. After this process, a similar voltammogram was obtained, with only a little decrease of peak current (Fig. s1b, see Supplementary material). This illustrated that rutin could be adsorbed onto the DNA-CPIE surface. Considering the signal of samples at CPIE without DNA modification was easily interfered by other substance [29], the DNA-CPIE was priority consideration for the determination of rutin in the experiment.
Influence of pH
The effect of pH on the response of rutin at the DNA-CPIE was studied. As can be seen, the peak current decreased gradually with increasing pH from 3.0 to 8.0 (results not shown). After pH exceeded 8.0, the peak nearly disappeared. It indicated that proton involved in the electrochemical reaction of rutin. In basic solutions, the peak current decreased gradually due to lack of proton. Synchronously, pH that was less than 3 may induce the alteration of DNA structure [30]. Therefore, pH 3.0 was selected as the optimal pH in the following experiments. Both Epa and E pc shifted negatively with the increase of pH. The relationships between the peak potential and pH were in the following: E pc (V) = 0.5879–0.052 pH, E 0’ (V) = 0.6318–0.056 pH, E pa (V) = 0.6757–0.060 pH (results not shown). From the slope value of the above equations, it could be concluded that E 0’ was pH dependent with a slope of 56 mV, which showed that the electron and proton ratio taking part in the electrode reaction was 1:1[31].
Influence of scan rate
The redox peak current increased linearly with scan rate (ν) from 0.01 to 0.3 V s−1 in pH 3.0 B-R buffer solution (Fig. 3). The linear regression equations were: ipa = –5.694 × 10−7–3.734 × 10−5 ν (ipa: A, ν: V s−1), ipc = 8.983 × 10−7 + 4.094 × 10−5 ν (ipc: A, ν: V s−1) (Fig. 3A). The result indicated that the redox process of rutin at the DNA-CPIE was controlled by adsorption. With the increase of ν, E pa shifted positively, E pc shifted negatively and ΔE p increased, indicating the reaction gradually became less reversible. From 0.08 to 0.3 V s−1, the peak potentials exhibited linear relationship with the logarithm of ν (Fig. 3B). The linear regression equations were: E pa (V) = 0.4117–0.020 lgν, E pc (V) = 0.5145 + 0.023 lgν. According to Laviron formula [32, 33], the charge transfer coefficient α was 0.54. Based on the equation: |E p–E p/2| = 47.7/αn [34], the electron transfer number n was calculated to be 2. Hence, the electrochemical redox mechanism of rutin involved two electrons and two protons process. As ∆ E p was more than 59/n mV and E p was dependent on ν, it could conclude that the redox reaction of rutin was a quasi-reversible process [34].
Influence of accumulation time and accumulation potential
The oxidative peak current increased with accumulation potential from −0.6 to 0.3 V, and then decreased rapidly (results not shown). Hence, 0.3 V was chosen as accumulation potential for subsequent analysis. The response of rutin increased with the increase of accumulation time. For 9.26 × 10−8 mol L−1 rutin, the peak current reached a maximum value after accumulation time was above 240 s. For 4.63 × 10−6 mol L−1 rutin, the curve leveled off after accumulation time exceeded 210 s (Fig. 4). So accumulation time 240 s was used in the further experiments.
Influence of the content of IL
Different content of IL was added into the mixture of carbon powder and paraffin oil in order to choose the most appropriate composite of paste electrode. As can been seen from Fig. 5, IL as the binder of the paste electrode enhanced the electrochemical response of rutin to some extent. When IL content increased from 0% to 5% (Fig. 5a → c), higher sensitivity and broader linear response for rutin could be observed. After adding more IL into the composites (for example 8%), the electrochemical response of the modified electrode decreased significantly (Fig. 5d). If the content of IL was beyond a certain value, the electrode exhibited a very large background current (results not shown). The high background current of DNA-CPIE may be due to the accessible capacitance of IL [28]. Replacing paraffin oil with IL as the binder, there was no peak that could be seen. It was in accordance with formerly reported [28]. So the optimal weight ratio of carbon powder: paraffin oil: IL was 80:15:5.
Analytical application
Differential pulse voltammetry (DPV) was employed for the determination of rutin at the DNA-CPIE (Fig. 6). Under the optimized experimental conditions, the oxidative current was linear with the concentration of rutin from 8 × 10−9 to 1 × 10−5 mol L−1. The linear regression equation was: ipa (A) = 2.90 × 10−8 + 0.28c (ipa: A, c: mol L−1). The linear range was wider than the value of 4.0 × 10−8 to 1.0 × 10−5 at the CPIE using 1-amyl-3-methylimidazolium bromide as modifier (1 × 10−8 mol L−1) [27]. The detection limit of 1.3 × 10−9 mol L−1 (S/N = 3), which was lower than the previous reports with SWNT-Au (1 × 10−8 mol L−1) [21]. The detection limit of rutin at the DNA-CPIE was not obvious lower than at the DNA-CPE. However, higher sensitivity and broader linear response for rutin at the DNA-CPIE could be observed comparing with at the DNA-CPE. The relative standard deviation (RSD) was 3.1% for ten successive determinations of 4.63 × 10−6 mol L−1 rutin using a single DNA-CPIE. The RSD was 8.2% with five separately prepared DNA-CPIEs. The stability of DNA-CPIE was also investigated. The biosensor was stored in the dry state in the refrigerator at 4°C when not in use. The oxidative current of 4.63 × 10−6 mol L−1 rutin on the biosensor could remain 96.8% of the initial response after one week use. These results indicated the DNA-CPIE had high sensitivity, good repreativity and stability.
DPV curves of rutin in B-R solution at the DNA-CPIE at different concentrations (a → n): 7.94 × 10−9, 1.0 × 10−8, 4.0 × 10−8, 7.99 × 10−8, 9.98 × 10−8, 3.97 × 10−7, 7.87 × 10−7, 9.8 × 10−7, 1.92 × 10−6, 3.70 × 10−6, 5.36 × 10−6, 6.9 × 10−6, 8.33 × 10−6, 1.09 × 10−5 mol L−1. Inset: Calibration plot of peak current vs. concentration of rutin
Rutin tablet marked 0.02 g per tablet was employed, and dissolved it with B-R buffer solution (pH 3.0) prior to use. Standard curve method was utilized to calculate the content three times, and the results were calculated as 0.02 ± 0.0004 g. It indicated that the results were consistent with the standard content. Table 1 showed that the recoveries were between 99.3% and 101.3%, which testified that the method was applicable to quantitative determination of rutin.
The influence of some potentially interfering species on the detection of rutin was investigated. The criterion used for the presence of interference was 5% or greater change in the peak current of 1.0 × 10−6 mol L−1 rutin. It was founded that 1,000 folds of Na+, K+, Ba2+, NO −3 , Cl−, glucose, 100 folds of citric acid, 1 fold of folic acid, dopamine, adrenalin, and AA did not interfere with the analysis of rutin at the DNA-CPIE. However, in the same conditions, the CPIE was easily interfered by potentially interfering species. 1,000 folds Ba2+, 1 fold of adrenalin, and AA interfered in the determination of rutin at the CPIE. Therefore, the DNA-CPIE was priority consideration for the determination of rutin in the experiment.
Conclusion
The DNA-CPIE was designed and the electrochemical behaviors of rutin were investigated. The electrochemical mechanism of rutin at the DNA-CPIE was an adsorption-controlled quasi-reversible process involving two electrons and two protons. DPV signal of rutin increased linearly with its concentration in the range from 8 × 10−9 to 1 × 10−5 mol L−1, with the detection limit of 1.3 × 10−9 mol L−1. It was found that the DNA-CPIE had long linear range and low detection limit for the determination of rutin. The excellent results indicated the method could be applied for the determination of other flavonoid compounds.
References
Zhao F, Wu X, Wang MK, Liu Y, Gao LX, Dong SJ (2004) Electrochemical and bioelectrochemistry properties of room-temperature ionic liquids and carbon composite materials. Anal Chem 76:4960–4967
Wang SF, Xiong HY, Zeng QX (2007) Design of carbon paste biosensors based on the mixture of ionic liquid and paraffin oil as a binder for high performance and stabilization. Electrochem Commun 9:807–812
Mai NN, Liu XY, Zeng XD, Xing L, Wei WZ, Luo SL (2010) Electrocatalytic oxidation of the reduced nicotinamide adenine dinucleotide at carbon ionic liquid electrode modified with polythionine/multi-walled carbon nanotubes composite. Microchim Acta 168:215–220
Kubisa P (2004) Application of ionic liquids as solvents for polymerization processes. Prog Polym Sci 29:3–12
DiCarlo CM, Compton DL, Evans KO, Laszlo JA (2006) Bioelectrocatalysis in ionic liquids examining specific cation and anion effects on electrode-immobilized cytochrome c. Bioelectrochem 68:134–143
Heitzman H, Young BA, Rausch DJ, Rickert P, Stepinski DC, Dietz ML (2006) Fluorous ionic liquids as solvents for the liquid–liquid extraction of metal ions by macrocyclic polyethers. Talanta 69:527–531
Maleki N, Safavi A, Tajabadi F (2006) High-performance carbon composite electrode based on an ionic liquid as a binder. Anal Chem 78:3820–3826
Safavi A, Maleki N, Tajabadi F (2007) Highly stable electrochemical oxidation of phenolic compounds at carbon ionic liquid electrode. Analyst 132:54–58
Sun W, Yang MX, Gao RF, Jiao K (2007) Electrochemical determination of ascorbic acid in room temperature ionic liquid BPPF6 modified carbon paste electrode. Electroanalysis 19:1597–1602
Rauf S, Gooding JJ, Akhtar K, Ghauri MA, Rahman M, Anwar MA, Khalid AM (2005) Electrochemical approach of anticancer drugs−DNA interaction. J Pharm Biomed Anal 37:205–217
Sun W, Li YZ, Gao HW, Jiao K (2009) Direct electrochemistry of double stranded DNA on ionic liquid modified carbon paste electrode. Microchim Acta 165:313–317
Pandey PC, Weetall HH (1994) Application of photochemical reaction in electrochemical detection of DNA intercalation. Anal Chem 66:1236–1241
Wang J, Chicharro M, Rivas G, Cai XH, Dontha N, Farias PAM, Shiraishi H (1996) DNA biosensor for the detection of hydrazines. Anal Chem 68:2251–2254
Gene RM, Cartana C, Adzet T, Marin E, Parella T, Canigueral S (1996) Anti-inflammatory and analgesic activity of baccharis trimera: identification of its active constituents. Planta Med 62:232–235
Ren WY, Qiao ZH, Wang HW, Zhu L, Zhang L (2003) Flavonoids: promising anticanceragents. Med Res Rev 23:519–534
Robards K, Antolovich M (1997) Analytical chemistry of fruit bioflavonoids. Analyst 122:11R–34R
Sun Y, Guo T, Sui Y, Li FM (2003) Quantitative determination of rutin, quercetin and adenosine in flos carthami by capillary electrophoresis. J Sep Sci 26:1203–1206
Ishii K, Furuta T, Kasuya Y (2001) Determination of rutin in human plasma by high-performance liquid chromatography utilizing solid-phase extraction and ultraviolet detection. J Chromatogr B 759:161
Song ZH, Hou S (2002) Sensitive determination of sub-nanogram amounts of rutin by its inhibition on chemiluminescence with immobilized reagents. Talanta 57:59–67
Legnerova Z, Satinsky D, Solich P (2003) Using on-line solid phase extraction for simultaneous determination of ascorbic acid and rutin trihydrate by sequential injection analysis. Anal Chim Acta 497:165–174
Zeng BZ, Wei SH, Xiao F, Zhao FQ (2006) Voltammetric behavior and determination of rutin at a single-walled carbon nanotubes modified gold electrode. Sens Actuators B 115:240–246
Wu SH, Sun JJ, Zhang DF, Lin ZB, Nie FH, Qiu HY, Chen GN (2008) Nanomolar detection of rutin based on adsorptive stripping analysis at single-sided heated graphite cylindrical electrodes with direct current heating. Electrochim Acta 53:6596–6601
Lewandowski A, Galin’ski M (2004) Carbon–ionic liquid double-layer capacitors. J Phys Chem Solids 65:281–286
Laszlo JA, Compton DL (2001) α-Chymotrypsin catalysis in imidazolium-based ionic liquids. Biotechnol Bioeng 75:181–186
Xiong HY, Chen T, Zhang XH, Wang SF (2007) High performance and stability of a hemoglobin-biosensor based on an ionic liquid as nonaqueous media for hydrogen peroxide monitoring. Electrochem Commun 9:2671–2675
Tian X, Li FJ, Zhu L, Ye BX (2008) Study on the electrochemical behavior of anticancer herbal drug rutin and its interaction with DNA. J Electroanal Chem 621:1–6
Zhang Y, Zheng JB (2008) Sensitive voltammetric determination of rutin at an ionic liquid modified carbon paste electrode. Talanta 77:325–330
Liu HT, He P, Li ZY, Sun CY, Shi LH, Liu Y, Zhu GY, Li JH (2005) An ionic liquid-type carbon paste electrode and its polyoxometalate-modified properties. Electrochem Commun 7:1357–1363
Li XL, Chen YL, Huang XY (2007) Electrochemical behavior of neomycin at DNA-modified gold electrodes. J Inorg Biochem 101:918–924
Duggan EL, Stevens VL, Grunbaum BW (1957) Deformation of deoxyribonucleate. I. titration and optical absorption studies of the effect of temperature, ionic strength and pH on DNA structure1. J Am Chem Soc 79:4859–4863
Duan LS, Xie F, Zhou F, Wang SF (2007) The electrochemical behavior of acetaminophen on multi-walled carbon nanotubes modified electrode and its analytical application. Anal Lett 40:2653–2663
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Bard AJ, Faulkner LR (1980) Electrochemical Methods, 2nd edn. Wiley, New York, 211
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 20875023).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 111 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Xiong, H., Zhang, X. et al. Detection of rutin at DNA modified carbon paste electrode based on a mixture of ionic liquid and paraffin oil as a binder. Microchim Acta 170, 27–32 (2010). https://doi.org/10.1007/s00604-010-0380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0380-7