Abstract
Selective determination of Ag(I) ion was accomplished based on the red-shift of the emission band of quantum dots (QDs). Under optimal conditions, a linear relationship does exist between the red-shift of the emission and the concentration of Ag(I) in the range from 1.0 × 10−7 to 1.5 × 10−5 mol L−1, with a detection limit of 5.0 × 10−8 mol L−1. The method has been successfully applied to the determination of Ag(I) ion in water samples. The possible reaction mechanism was investigated by ultraviolet–visible absorption, fluorescence, Raman spectroscopies and by high resolution transmission electron microscopy. The results suggest that the red-shift in emission be attributed to the stabilization of a charge-transfer state, but not due to the aggregation induced by AgI(I) ion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past two decades, the development of metal ion fluorescent sensors continues to grow at an unbated pace [1]. So far, there have been several reports on optical based detection of metal ions using fluorescent small molecules (organic dyes) [2]. However, most of them usually suffer from serious drawbacks such as weak signal intensities and photobleaching. Furthermore, these dye small molecules tend to display a narrow excitation followed by a broad emission band with red tailing [3, 4].
Quantum dots (QDs, also known as semiconductor nanocrystals) have attracted great interest in developing fluorescent sensors for metal ions owing to their unique and fascinating optical properties [5, 6]. Up to now, several groups have employed them as ion probes [7–18]. Chen’s group first utilized CdS QDs modified with different organic ligands for the determination of Cu2+ and Zn2+ in aqueous media [7]. Subsequently, Gattás-Asfura and co-workers demonstrated that the fluorescence intensity of peptide-coated CdS QDs was reduced selectively in the presence of Cu2+ and Ag+ [8]. And then, a chemodosimeter for Ag+ was designed in the system of BSA absorption on the CdSe QDs modified with thioglycolic acid (TGA) [9]. Recently, our group reported a novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe QDs [13], and was successfully applied to the analysis of Pb2+ in food samples. These QDs based sensors mainly focus on the specific surface ligands for sensing different metal ions, as well as the great fluorescence intensity and stability against photobleaching. However, the ligand functionalized process is time-consuming and laborious, and in many cases, ligands disturb detection sensitivity and selectivity. Additionally, lack of selectivity is the major problem reported for some of the procedures proposed and most of sensing mechanisms are based on quenching or enhancing of fluorescence intensity of QDs.
In the present work, we report a new and simple method for selective detection of Ag+ based on the red-shift of emission wavelength of QDs in aqueous media. As expected, the experimental results show that the red-shift of emission wavelength is linearly proportional to the concentration of Ag+ with high sensitivity and selectivity, other potentially interfering ions, including Cu2+ and Hg2+, can hardly cause extra red-shift of emission wavelength in the present of Ag+. Unlike the previous approaches for detection of metal ions by quenching or enhancing of fluorescence intensity of QDs, we have demonstrated a red-shift of emission wavelength based CdTe QDs probe. To the best of our knowledge, this describes the first example to detect metal ions based on the red-shift of emission wavelength of QDs. The present method is successfully applied to the determination of Ag+ in water samples. In addition, the possible red-shift of emission wavelength mechanism is also described.
Experimental
Apparatus
The ultraviolet–visible (UV–vis) absorption spectra were acquired on a Thermo Nicolet Corporation Model evolution 300 spectrophotometer (Nicolet, UK, http://www.nicolet.co.uk/) coupled with a 1.00 cm quartz cell. The fluorescence spectra were performed on a Perkin Elmer Model LS-55 luminescence spectrometer (Perkin Elmer, USA, http://www.perkinelmer.com/) equipped with a 20 kW xenon discharge lamp as a light source. The excitation wavelength was 380 nm. The slit width for excitation was 15 nm and the slit width for emission was 2.5 nm. Dilute solutions of QDs in aqueous medium were placed in 500 μL quartz cuvettes and their corresponding fluorescence was measured at room temperature. The Raman spectra were performed with an inVia Raman spectroscopy system (Renishaw, UK, http://www.renishaw.com/en/6150.aspx/), equipped with a He–Ne laser excitation source emitting wavelength at 633 nm. The high resolution transmission electron microscopy (HR-TEM) images of the QDs were acquired on a JEM-2010FEF transmission electron microscope (Jeol, Japan, http://www.jeol.com/).
Reagents
Acetone, 3-hydroxymethyl-methane (Tris), hydrochloric acid, sodium chloride and sodium hydroxide were purchased from Shanghai Boao Biotechnology Co, Ltd (Shanghai, China, http://biotechnologycoltd.itm.com.cn/). CdCl2·2.5H2O (99.0%), tellurium powder (99.99%) and NaBH4 (96%) were obtained from Tianjin Chemical Reagent Plant (Tianjin, China, http://www.reagent-1.com/product_en/product2e.htm). The 0.1 mol L-1 phosphate buffer solutions (PBS) were adjusted by changing the ratio of Na2HPO4 to NaH2PO4 and the 0.1 mol L−1 Tris-HCl buffer solutions were adjusted by changing the ratio of Tris to HCl. TGA was obtained from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China, http://www.reagent.com.cn/corporation/infoDetail.asp?dInfoId=136). All other reagents were of analytical grade and doubly deionized water was used throughout.
Preparation of TGA-capped CdTe QDs
The TGA-capped CdTe QDs were synthesized according to the method reported previously with minor modifications [19]. Tellurium powder was used to prepare NaHTe aqueous solution. Briefly, 25.0 mL 0.01 mol L−1 CdCl2 was transferred to a 100 mL flask bubbled with N2 for 30 min. Subsequently, this solution was mixed with 0.1 mL TGA at N2 atmosphere. At this point, the pH of the mixture was adjusted to 11.0 by dropwise addition of 1.0 mol L−1 NaOH solution. Then NaHTe aqueous solution was injected into the above mixture swiftly. Finally, 25.0 mL this mixture kept heating at 100 °C under vigorous stirring for 30 min. The crude products were precipitated by acetone with centrifugation at 8,000 rpm for 5 min. The resultant precipitate was redispersed in doubly deionized water, and then kept in dark at 4 °C for further use.
Standard procedures for spectrofluorometric detection of Ag+
In a 5.0 mL calibrated test tube 1.0 mL 5.0 × 10−6 mol L−1 TGA-capped CdTe QDs solution was added and certain amounts of Ag+ were sequentially transferred. The mixture was then diluted to volume with doubly deionized water and mixed thoroughly. The red-shift of emission wavelength of the prepared colloidal solution was recorded with the excitation wavelength of 380 nm.
Results and discussion
Characterization of water-soluble TGA-capped CdTe QDs
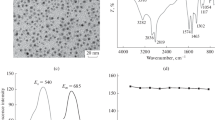
A typical UV–vis absorption and fluorescence spectra for the TGA-capped CdTe QDs are shown in Fig. 1. Acoording to the UV–vis absorption peak (496 nm), the size of QDs is estimated as 2.2 nm [20, 21]. In addition, Fig. 1b displays obvious and symmetrical fluorescence emission spectra with an emission maximum at 530 nm. It could be seen that the full-width at half-maximum (fwhm) of the fluorescence spectra is narrow, which indicates that the as-prepared CdTe QDs are nearly monodisperse and homogenous. The TGA-capped CdTe QDs are futher characterized by HR-TEM (Fig. 2a). The results show that the CdTe QDs possess good crystalline structures, and the size of QDs is consistent with that calculated from the absorption spectra. The HR-TEM image (Fig. 2b) reveals the QDs are also monodiserse with slightly increasing on particlesize after adding 1.0 × 10−4 mol L−1 Ag+.
Effect of reaction time
Initial experiments demonstrate that the reaction between TGA-capped CdTe QDs and Ag+ is completed within 5 min and the fluorescence signals are stable for more than 30 min. The experimental data were recorded after the reaction lasted for 15 min.
Effect of pH
The effect of pH on the red-shift of emission wavelength of QDs has been carefully investigated. As shown in Fig. S-1 (Electronic Supplementary Material), with increasing Ag+ concentration, the red-shift of emission wavelength of TGA-capped CdTe QDs first increase and then decrease, and the maximum change of red-shift occurred when pH is close to 8. Although the red-shift in optimum pH buffer solution is near to that in the pure aqueous solution at the same concentration of Ag+, it is found that the relative red-shift of emission wavelength of TGA-capped CdTe QDs and the corresponding concentration of Ag+ are not proportional in both the PBS solutions (Fig. S-2) and Tris-HCl buffer solutions (Fig. S-3). However, a good linear relationship is observed in the pure aqueous solution (Fig. 3b). So, the pure aqueous solution was recommended for use.
Effect of TGA-capped QDs concentration
In order to acquire the highest linear correlation coefficient and the widest linear range on this system, the influence of the concentration of TGA-capped CdTe QDs on red-shift of emission wavelength of TGA-capped QDs was studied. Due to the optimal concentration of CdTe QDs can not be determined only by comparing the change of the red-shift, so the linear equations of three different concentrations of CdTe QDs are integrated to choose the optimal concentration of CdTe QDs. As shown in Table S-1, three different linear relationships under different concentrations of TGA-capped CdTe QDs are obtained. For a compromise between linear correlation coefficient and the linear range, 1.0 × 10−6 mol L−1 TGA-capped CdTe QDs solution was recommended.
Calibration and sensitivity
On the basis of the aforementioned experimental results, the fluorescence spectra of TGA-capped CdTe QDs under different concentrations of Ag+ were recorded at optimum experimental conditions. The results are shown in Fig. 3. The first observed fluorescence wavelength centered at 530 nm, with the addition of different concentrations of Ag+ solutions, the fluorescence emission peak shifted to longer wavelength gradually. From the results obtained so far, an expression that relates Ag+ with fluorescence emission wavelength maximum can be written as following:
Y is the red-shift of emission wavelength of the TGA-capped CdTe QDs in presence and absence of Ag+, [Ag+] represents the equilibrium concentration of Ag+, a is the slope of the calibration curve which could be calculated by standard curve, and b is the intersection at the y axis. A very good linear relationship (R 2 = 0.9942) is observed up to Ag+ concentration ranging from 1.0 × 10−7 to 1.5 × 10−5 mol L−1 (Fig. 3b), and the detection limit is 5.0 × 10−8 mol L−1.
Effect of potentially interfering ions
Following the traditional procedure, the fluorescence spectra of TGA-capped CdTe QDs with various potentially interfering ions were conducted to examine the selectivity. As shown in Table 1, the interference of alkali, alkaline earth ions, Co2+, Mn2+ and Ni2+ are very weak. Cd2+, Mg2+, Zn2+, Pb2+ and Ba2+ have a relative fluorescence wavelength blue-shift effect on TGA-capped CdTe QDs at relative higher concentration. Cu2+, Hg2+ also reveal certain red-shift on TGA-capped CdTe QDs fluorescence wavelength; however, when the same concentration of Cu2+ or Hg2+ are added in the QDs-Ag+ system, it is found that the red-shift of emission wavelength of TGA-capped CdTe QDs has no obvious increment in comparison with the red-shift caused by Ag+ (Fig. S-4). At these conditions, the red-shift may be mainly attributed to the interaction between Ag+ and QDs, and the effects of Cu2+ and Hg2+ on red-shift are ignored. Halogen anions, CO 2−3 , SO 2−4 , SO 2−3 , NO −3 etc. familiar anions have hardly effect on QDs fluorescence emission wavelength. The obtained results demonstrate that this approach has a better selectivity.
Mechanism of reaction
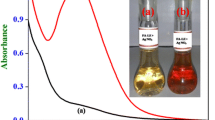
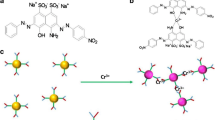
Investigation of the reactions of CdTe QDs with Ag+ have attracted much attention in the past few years. Several mechanisms were proposed to explain the process of Ag+ enchaning or quenching fluorescence of QDs [22–26], for example, Dong and co-workers suggested that Ag+ formed bonds with Te on the QD surface[23]. On the other hand, Son [22] and Leung [24] reported cation exchange between Ag+ and Cd2+ on CdTe tetrapods and CdSe QDs surface, respectively. In our experiment, whether or not the reaction involving AgTe structure which was formed on the surface of QDs was validated through the UV–vis absorption spectra (Fig. 4) and Raman spectra (Fig. 5). Firstly, it is found that the slight increment in the absorbance of the CdTe solution and the absorption onsets display a gradual red shift, suggesting the formation of AgTe structure on QD surface after addition of Ag+ [26, 27]. In comparison with the Raman spectra of CdTe QDs in absence of Ag+, a new peak which confirmed the existen of AgTe is observed. To further investigate the reaction mechansim of Ag+ with CdTe QDs, the effect of surface ligand (TGA) concentration is illustrated in Fig. S-5. It is obvious that the red-shift of emission wavelength is not linearly proportional to the concentration of Ag+ when excessive TGA is added into the system. It indicates that Ag+ could not only bind to the surface of CdTe QDs, but also coordinate with the thiols on the surface of CdTe QDs, due to its strong affinity towards RSH and RS− compounds [24, 25]. On the basis of above-mentioned results, we conclude the process of reaction in Scheme 1. As shown in Scheme 1, the particlesize is slightly increased by AgTe structure and Ag-SR salt formed on the surface of QDs, which is also observed under the HR-TEM (Fig. 2b). These results suggest that the red-shift in emission may be attributed to the stabilisation of chargetransfer state which is expected to conver the surface to AgTe and Ag-SR salt, not due to the agglutination induced by Ag+. To confirm this hypothesis, we next examine the fluorescence spectra of two different sizes of TGA-capped CdTe QDs solution mixed with Ag+. As can be seen in Fig. S-6, on one hand, it exhibits that with the increase of the concentration of Ag+, two different representative sizes of CdTe QDs fluorescence wavelength shift to longer wavelength in the corresponding; on the other hand, it can be observed that at increasing Ag+ concentrations the fluorescence signals first increase and then decrease. Mechanism study shows that small QDs with more traps on the particle surface are effectively passivated by initial adsorbed Ag+, which accounts for the fluorescence enhancement observed; after the initial traps are saturated, the excess Ag+ facilitates nonradiative recombination, resulting in fluorescence quenching [26]. These phenomenons could be interpreted as the fact that the reactions between TGA-capped CdTe QDs and Ag+ are independent, not to lead the agglutination of QDs.
Analytical application
To evaluate the applications of the method, it was used to the determination of Ag+ in mineral water and pond water samples. The samples were analyzed by standard addition method. The results are summarized in Table 2. From the Table 2 it can be seen that recovery and the relative standard deviation for two samples are satisfactory, suggesting that the proposed mothod is reliable and practical.
Conclusions
In summary, a simple and specific strategy, based on the red-shift of emission wavelength of TGA-capped CdTe QDs, has been successfully developed for the detection of Ag+ with remarkably high selectivity and sensitivity. The red-shift in emission probably is attributed to the creation of more radiative centers or more new defects by complex of Ag+ and TGA formed on the surface of CdTe QDs. To the best of our knowledge, this is the first example to detect metal ions based on the red-shift of emission wavelength of QDs. Moreover, the analytical results of real samples are satisfactory. Our preliminary results inspire that the fluorescence QDs probes based red-shift of emission wavelength will become a promising attractive alternative probes in detecting metal ions and biological macromolecules.
References
Rurack K, Kollmannsberger M, Resch-Genger U, Daub J (2000) A selective and sensitive fluoroionophore for HgII, AgI, and CuII with virtually decoupled fluorophore and receptor units. J Am Chem Soc 122:968
Yang RH, Chan WH, Lee AWM, Xia PF, Zhang HK, Li KA (2003) A ratiometric fluorescent sensor for AgI with high selectivity and sensitivity. J Am Chem Soc 125:2884
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013
Mattoussi H, Manro JM, Goldman ER, Anderson GP, Sundar VC, Mikula FV, Bawendi MG (2000) Self-assembly of CcuhdSe–ZnS quantum dot bioconjugates using an engineered recombinant protein. J Am Chem Soc 122:12142
Chan WCW, Nie SM (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016
Han MY, Gao XH, Su JZ, Nie SM (2001) Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol 19:631
Chen YF, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132
Gattás-Asfura KM, Leblanc RM (2003) Peptide-coated CdS quantum dots for the optical detection of copper(II) and silver(I). Chem Commun 21:2684
Liang JG, Ai XP, He ZK (2004) Functionalized CdSe quantum dots as selective silver ion chemodosimeter. Analyst 129:619
Banerjee S, Kar S, Santra S (2008) A simple strategy for quantum dot assisted selective detection of cadmium ions. Chem Commun 26:3037
Xia YS, Zhu CQ (2008) Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 75:215
Xu C, Bakker E (2007) Multicolor quantum dot encoding for polymeric particle-based optical ion sensors. Anal Chem 79:3716
Wu HM, Liang JG, Han HY (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim Acta 161:81
Chen JL, Zhu CQ (2005) Functionalized cadmium sulfide quantum dots as fluorescence probe for silver ion determination. Anal Chim Acta 546:147
Shang ZB, Wang Y, Jin WJ (2009) Triethanolamine-capped CdSe quantum dots as fluorescent sensors for reciprocal recognition of mercury (II) and iodide in aqueous solution. Talanta 78:364
Lai SJ, Chang XJ, Fu C (2009) Cadmium sulfide quantum dots modified by chitosan as fluorescence probe for copper (II) ion determination. Microchim Acta 165:39
Zhang LJ, Xu CL, Li BX (2009) Simple and sensitive detection method for chromium(VI) in water using glutathione-capped CdTe quantum dots as fluorescent probes. Microchim Acta 166:61
Chen HQ, Liang AN, Wang L, Liu Y, Qian BB (2009) Ultrasensitive determination of Cu2+ by synchronous fluorescence spectroscopy with functional nanoparticles. Microchim Acta 164:453
Xia Y, Zhang T, Diao X, Zhu C (2007) Measurable emission color change: size-dependent reversible fluorescence quenching of CdTe quantum dots by molecular oxygen. Chem Lett 36:242
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854
Schmelz O, Mews A, Basché T, Herrmann A, Müllen K (2001) Supramolecular complexes from CdSe nanocrystals and organic fluorophors. Langmuir 17:2861
Son DH, Hughes SM, Yin YD, Alivisatos AP (2004) Cation exchange reactions in ionic nanocrystals. Science 306:1009
Dong CQ, Qian HF, Fang NH, Ren JC (2006) Study of fluorescence quenching and dialysis process of CdTe quantum dots, using ensemble techniques and fluorescence correlation spectroscopy. J Phys Chem B 110:11069
Leung LK, Komplin NJ, Ellis AB, Tabatabaie NJ (1991) Photoluminescence studies of silver-exchanged cadmium selenide crystals: modification of a chemical sensor for aniline derivatives by heterojunction formation. Phys Chem 95:5918
Tamar D, Nikolai G, Alexander E, Daniel M (2008) Studying the reactions of CdTe nanostructures and thin CdTe films with Ag+ and AuCl −4 . J Phys Chem C 112:8881
Xia YS, Cao C, Zhu CQ (2008) Two distinct photoluminescence responses of CdTe quantum dots to Ag (I). J Lumin 128:166
Isarov AV, Chrysochoos J (1997) Optical and photochemical properties of nonstoichiometric cadmium sulfide nanoparticles: surface modification with copper(II) ions. Langmuir 13:3142
Acknowledgements
The authors gratefully acknowledge the support for this research by National Natural Science Foundation of China (20675034), the Program for academic pacesetter of Wuhan (200851430484), Nature Science foundation key project from Hubei Province of China (2008CDA080), the Youth Chengguang Project of Science and Technology of Wuhan City of China (200850731359) and Natural Science Foundation of Hubei Province (No.2008CDB031).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 100 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Liang, J., Sheng, Z. et al. A novel strategy for selective detection of Ag+ based on the red-shift of emission wavelength of quantum dots. Microchim Acta 167, 281–287 (2009). https://doi.org/10.1007/s00604-009-0244-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0244-1