Abstract
Multiwalled carbon nanotubes were used as solid phase extraction (SPE) adsorbent for the determination of four chloroacetanilide herbicides (alachlor, acetochlor, metolachlor and butachlor) in water. The primary factors that influence the efficiency of the SPE performance, such as the amount of the adsorbent, the eluent solvent, the pH and the sample volume, were investigated and optimized. Under optimized conditions, the recoveries of the four herbicides at three spike levels were in the range 76.7–104.4%, and the RSDs ranged from 2.5–12.7%. Good linearity was obtained for the pesticides in the concentration range 0.0025–2.5 mg L−1, and the detection limits were 0.01–0.03 μg L−1 at signal-to-noise ratios of 3:1. The method was successfully applied to the determination of these analytes in tap water and river water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During last decade, herbicides were widely produced and used in the world, and many reports have reported about the herbicides residues in crop and environment [1–3]. Herbicides residues in water also aroused much attention because they can be accumulated via the food chain and do harm to the environment and human health. In order to control the potential toxicity of herbicides, many countries enact correlative environmental laws. An EU directive stated that the pesticide level in drinking water should not exceed 0.1 μg L−1 for individual compounds and some of their degradation products, and should not exceed 0.5 μg L−1 for the sum of all compounds [4].
As one group of the most primary herbicides, chloroacetanilide herbicides were commonly used to control weeds in various crops, such as corn, wheat, soybean and so on. In recent years, the environmental problem caused by this kind of herbicides was gradually emerged [5]. It was reported that chloroacetanilide herbicides and their metabolites can induce sister chromatide exchanges in cultured human lymphocytes [6]. For acetochlor, it can carry strong genotoxicity activity in vitro [7]. Butachlor was a suspected carcinogen capable of stimulating cell proliferation and inducing malugnant transformation in vitro [8]. These herbicides are so harmful that it should be severely controlled and monitored in environment. It was pity that there was no correlative standard or quality criteria for these herbicides in water, for example, the water standards or quality criteria for butachlor and acetochlor were not yet established. So it was very useful and significant to develop the analysis method to detect and monitor these compounds in environment water. In this article four representive herbicides (alachlor, acetochlor, metolachlor and butachlor) were selected, which were belonging to chloroacetanilide family as the main part and had substantive production in china [9–11], and the residue analysis method for them in water was studied.
The way for enriching and extracting this kind of compounds from environmental water contained various techniques, including liquid–liquid extraction (LLE), solid-phase extraction (SPE) and micro-extraction technology, such as liquid-phase micro-extraction (LPME) [12, 13] or solid-phase microextraction (SPME) [14]. Comparing with LLE and the microextraction methods, SPE was used more widely because it was simple, credible, and can realize full extraction. In recent years, mutilwalled carbon nanotube (MWCNT), a new nanoscale material, has received extensive concern in environmental field [15]. With attractive electronic, chemical, and mechanical properties it has been widely used in the construction of adsorbents, sensors and nano-scale electronic devices [16]. As a new kind of potent adsorbent, the MWCNT has been successfully used for the extraction of triazine herbicides [17], OPPs [18, 19], sulfonylurea herbicides [20], and DDTs [21] from water samples. The research found out that MWCNT showed excellent extraction efficiency in the procedure of SPE because of its trait of nanometer material, such as its large specific surface areas. In these reports, besides that GC-FPD was used to detect OPPs, HPLC was used as the detection instrument in most of the cases.
The purpose of this article was to investigate the extraction efficiency of MWCNT as a new SPE adsorbent for the analysis of chloroacetanilide herbicides (alachlor, acetochlor, metolachlor and butachlor), and to establish a simple, credible and sensitive method for detecting the four chloroacetanilide herbicides in water at trace level. Considering of the predominant response of these herbicides, the GC-ECD was used as the detection technology in order to get high sensitivity.
Experimental
Reagents and materials
The standard herbicides alachlor (99.7%), acetochlor (98%), butachlor (97.5%) were obtained from Agricultural Environmental Protection Institution in Tianjin, China, and metolachlor (96%) was provided by Syngenta (Shanghai, China). The HPLC grade solvent, including acetone, ethyl–acetate were produced by Sigma–Aldrich company (Shanghai, China, http://www.sigmaaldrich.com). The distilled water was produced from the water distilling equipment (Keru Company, Shanghai, China). The methanol, n-hexane, acetone, hydrochloric acid, ammonia and anhydrous sodium sulphate were all analytical grades and supplied by Beijing Chemical Reagent Company (Beijing, China, http://www.crc-bj.com). The standard stock solution containing 1,000 μg mL−1 herbicides were prepared by dissolving an appropriate amount of the compounds in n-hexane.
The adsorbent material MWCNT (The average internal diameter of 3.5 nm; the average external diameter of 8.6 nm; the length of 0.5 ∼ 1,000 μm) was purchased from the Department of Chemical Engineering, Tsinghua University, Beijing, China. The analyzed water sample was drinking water from Wahaha Limited Group, Hangzhou, China.
Preparation of the MWCNT cartridge
The MWCNT cartridges were packed manually by modifying the Agilent ODS-C18 SPE cartridges (http://www.agilent.com). Evacuate the container of the cartridge, then the adsorption material was packed into the cartridge which was cleaned and dried, and then the MWCNT material was held in the cartridge with two pieces of 20-μm polypropylene.
Solid-phase extraction procedure
The MWCNT cartridge was pre-conditioned with 2 mL ethyl–acetate, 2 mL methanol and 2 mL of distilled water. Then the water sample was added. The passage of the water sample through the SPE cartridge was carried out at a flow-rate of 4 ∼ 5 mL min−1 under vacuum. After all of the water was passed through, the SPE cartridge was dried under vacuum for 5 min. Then 7 mL ethyl–acetate (4 mL+3 mL) was used as the eluent and the eluate was collected in a 10 mL curette. To get rid of the water, the eluate was passed through an anhydrous sodium sulphate filtrate (3 g), and then the eluate was dried by rotary-evaporation in 30°C water bath. Finally the residue was redissolved in 1 mL n-hexane for GC analysis.
Gas chromatography
An Agilent-6890N gas chromatography equipped with micro-electron-capture detector (μECD) was used to detect the herbicides. The instrument was equipped with the fused-silica capillary column: HP-5, 30 m × 0.32 mm × 0.25 μm. The temperature of the injector and detector were kept at 290°C, and the carrier gas N2 were at flow rate of 1.0 mL min−1 at constant flow mode. A 1-μL sample was injected into the column at the splitless mode. The temperature of the oven was set as follows: initial 100°C (held for 1 min), 10°C min−1 to 200°C (held for 1 min), then 5°C min−1 to 240°C (held for 0.5 min), and finally 20°C min−1 to 260°C (held for 5 min). The chromatogram of the water sample was shown in Fig. 1.
The chromatogram of water sample (Four herbicides were fortified into 500 mL water sample, the fortified level were 0.5 μg L−1 for acetochlor and alachlor, and 1.0 μg L−1 for metolachlor and butachlor.). Retention times were: acetochlor 13.9 min, alachlor 14.2 min, metolachlor 15.2 min and butachlor 18.2 min
Results and discussion
Optimization of the SPE procedure
In order to investigate the performance of MWCNT as a SPE adsorbent for enrichment of the analytes from water, the main experiment conditions affecting the extraction efficiency such as the eluent and its volume, the amount of adsorbent, the pH of water sample and sample volume were investigated.
Effect of the polarity and volume of eluent solvent
The selection of eluent and its volume played an important role for the SPE procedure, which ensured that the object targets can be eluted completely from the SPE cartridge. In order to select an appropriate eluent, four solvents (methanol, acetone, ethyl–acetate and n-hexane) and five different volumes (2 mL, 4 mL, 6 mL, 8 mL, and 10 mL) were used. 200 mL water sample (fortified with alachlor: 2.5 μg L−1, acetochlor: 2.5 μg L−1, metolachlor: 5.0 μg L−1, butachlor: 5.0 μg L−1) were passed through the cartridge packed with 100 mg MWCNT, and the fortified recoveries were investigated.
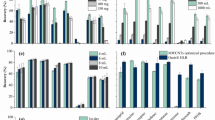
The change trends for the recoveries of herbicides at different eluent volumes were shown in Fig. 2 and Figs. S1, S2, and S3. It was found that ethyl–acetate was the best eluent for the four herbicides, and the average recoveries were from 83.4% (alachlor) to 92.3% (butachlor) after eluted with 6 mL ethyl–acetate. The other three solvents also eluted part of the target objects, but the recoveries were less than 65.0%. Maybe because of the low polarity, after eluted with 10 mL n-hexane, the recoveries did not exceed 53.0%. For the methanol and acetone, the recoveries were a little higher, but still did not exceed 65.0%. All the recoveries were above 80% after eluted with 6 mL of ethyl–acetate, and the recoveries changed little when the volume of ethyl–acetate was increased. So ethyl–acetate was chosen as the eluent solvent and the volume was 7 mL to ensure the full elution.
Effect of the weight of adsorption material
As an adsorption material, its adsorption capability was an important property. The effects of the amount of adsorbent on the recoveries were examined in the range of 50 ∼ 150 mg. The results were shown in Table 1. When the MWCNT amount was 50 mg, the recoveries for the four herbicides were in the range of 68.2% to 72.0%. As the amount increased to 100 mg, the recoveries increased to 84.2% (acetochlor), 83.4% (alachlor), 87.1% (metolachlor) and 93.2% (butachlor) respectively. When the amount sequentially increased to 150 mg, the recoveries changed little. So 100 mg of MWCNT was chose as the amount of the MWCNT for the method.
Effect of sample pH
Sample pH played an important role in the enrichment of target objects from water, because the pH decided and influenced the state of the herbicides in water, and change of pH can affect the efficiency of extraction. Considering that the pH values of water samples in the environment were usually between 3–9, 4 pH values (3.0, 5.0, 7.0, 9.0) were chosen. 1 mol L−1 hydrochloric acid and 1 mol L−1 ammonia were used to adjust the pH of water samples. The pH value of the water samples were measured using a pH meter (PHS-2C model, produced by Shuoguang Electrical Company, Shanghai, China). The recoveries of the herbicides at different pH were shown in Fig. 3. The results showed that the recoveries were mostly in the range of 66.5–78.4% when the sample pH was 3, 5 and 9 (except that the recovery of butachlor was 93.7% when the sample pH was 5). When the sample pH was 7, the recoveries were much better, which were above 83.4%, and its RSDs were in the range of 4.7–5.2%. The results showed that the pH of water samples can affect the adsorption and elution efficiency of the MWCNT material. Only when the sample was neutral, good recoveries can be obtained. So pH 7 was selected as the optimum of the water sample.
Effect of sample volume
The effect of the different sample volume on the recoveries was also investigated. As the amount of each pesticide was set as: alachlor: 0.5 μg, acetochlor: 0.5 μg, metolachlor: 1.0 μg, butachlor: 1.0 μg, and the sample volume changed from 200 mL to 1,000 mL, the recoveries were studied. The results in Fig. S4 showed that the MWCNT was a good adsorption material for the four herbicides. When the sample volume changed from 200 mL to 1,000 mL, the recoveries changed little and all the recoveries were in the range of 80.8% to 93.2%. It showed that the change of sample volume from 200 mL to 1,000 mL did not affect the recoveries of the four herbicides. Because the concentration of herbicides in water samples was usually very low and the provision for the herbicides in water was very strict (usually not exceed 0.1 μg L−1), we usually chose a larger sample volume to get a lower detection limit. But the volume of water sample was usually below 1,000 mL, because it would cost much long time for the water to get through the cartridge. So 500 mL was chose as the sample volume in order to obtain a better enrichment factor and save time.
Detection limits, precision and linear range
The accuracy, precision, sensitivity and the linear range were important and indispensable parameters for an analytical method. Under the optimal conditions, all the parameters of the analytical method were validated. As shown in Table 2, the fortified recoveries of the four herbicides at three adding levels were mostly above 80%, in the range of 81.2–104.4% (except that the recovery of butachlor was 76.7%, when the adding level was 0.1 μg L−1), and the RSDs were in the range of 2.5–12.7%. These were all acceptable for a residue analytical method. The linear ranges for the four pesticides were shown in Table 3. The results showed that the GC-ECD responses expressed high sensitivity and fine linear relationship over the concentrations of the four pesticides in the range of 0.0025 mg L−1 to 2.5 mg L−1, and the correlation coefficients were in the range of 0.994 to 0.998. The LOD values of the method were obtained by using control samples fortified with working standard at different levels; signal-to-noise ratios of 3:1 were the criteria for the LOD values. The limit of detection for the four herbicides were 0.02 μg L−1 for alachlor, 0.01 μg L−1 for acetochlor, 0.03 μg L−1 for metolachlor and 0.02 μg L−1 for butachlor respectively, which were lower than 0.1 μg L−1.
Application of the method on environmental water samples
Finally, the method was used to determine the four herbicides residue in tap water and river water in order to validate its application on the environmental water samples. Tap water was collected from a tap after flowing about 5 min from our laboratory in Beijing, and the river water was taken from Jingmi Irrigation Canal in Beijing, China. The four herbicides were not found in the tap water and the river water sample. Then the two kinds of environmental water samples (500 mL) were spiked with the four herbicides (alachlor: 1 μg L−1, acetochlor: 1 μg L−1, metolachlor: 2.0 μg L−1 and butachlor: 2.0 μg L−1) and the recoveries were evaluated. The results were listed in Table 4. The average recoveries of the four herbicides in the tap water and river water were in the range of 81.2–87.7%, and the RSD were 2.6–5.4%. It showed that the method can be applied to monitor the four herbicides in environmental water.
Conclusions
A fast, simple and sensitive analytical method with SPE enrichment and GC-ECD detection was developed for determination of alachlor, acetochlor, metolachlor and butachlor in environmental water, and the SPE performance of the multiwalled carbon nanotube as a novel adsorbent was evaluated. The MWCNT expressed good adsorptive capacity as an effective SPE adsorbent for enrichment of the herbicides in water.
Comparing with the commonly used adsorbents such as C18, the amount of adsorbent was much less for MWCNT. Only 100 mg MWCNT was used in this method, but normally 500 mg to 1,000 mg C18 cartridges were used in the environment analysis routine work. Moreover, normally MWCNT was cheaper than C18. So potentially MWCNT can be a widely used SPE adsorbent with the advantage of less using amount and cheap cost.
References
Blewetta TC, Robertsa DW, Brintona WF (2005) Phytotoxicity factors and herbicide contamination in relation to compost quality management practices. Renewable Agric and Food System 20:67
Xue ND, Xu XB, Jin ZL (2005) Screening 31 endocrine–disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir. Chemosphere 61:1594
Sakai M (2002) Use of chronic tests with Daphnia magna for examination of diluted river water. Ecotoxicol Environ Saf 53:376
Nogueira JMF, Sandrab T, Sandra P (2004) Multiresidue screening of neutral pesticides in water samples by high performance liquid chromatography–electrospray mass spectrometry. Anal Chim Acta 505:209
Liu SY, Chen YP, Yu HQ, Zhang SJ (2005) Kinetics and mechanisms of radiation—induced degradation of acetochlor. Chemosphere 59:13
Oosterhuis B, Vukman K, Vagi E, Glavinas H, Jablonkai I, Krajcsi P (2008) Specific interactions of chloroacetanilide herbicides with human ABC transporter proteins. Toxicology 248:45
Helbing CC, Ovaska K, Ji L (2006) Evaluation of the effect of acetochlor on thyroid hormone receptor gene expression in the brain and behavior of Rana catesbeiana tadpoles. Aquatic Toxic 80:42
Yakovleva J, Zherdev AV, Popova VA, Eremin SA, Dzantiev BB (2003) Production of antibodies and development of enzyme–linked immunosorbent assays for the herbicide butachlor. Anal Chim Acta 491:1
Yu YL, Chen YX, Luo YM, Pan XD, He YF, Wong MH (2003) Rapid degradation of butachlor in wheat rhizosphere soil. Chemosphere 50:771
Yao B, Xu JM, Zhang CL (2003) Behavior of herbicide butachlor in environment. Ecol Environ (Chinese) 12:66
Sheng J, Bai LY, Liu XY (2005) The review of the acetanilide herbicides and their safeners. Jiangxi Plant Prot 28:163
Lambropoulou DA, Albanis TA (2007) Liquid-phase micro-extraction techniques in pesticide residue analysis. J Biochem Biophys Methods 70:195
Zhao ER, Shan WL, Jiang SR, Liu Y, Zhou ZQ (2006) Determination of the chloroacetanilide herbicides in waters using single-drop microextraction and gas chromatography. Microchem J 83:105
Xu XQ, Yang HH, Wang L, Han B, Wang XR, Lee FS (2007) Analysis of chloroacetanilide herbicides in water samples by solid-phase microextraction coupled with gas chromatography-mass spectrometry. Anal Chim Acta 591:87
Merkoci A (2006) Carbon nanotubes: exciting new materials for microanalysis and sensing. Microchim Acta 152:155
Guo RX, Xu Q, Wang DY, Hu XY (2006) Trace determination of clenbuterol with an MWCNT-Nafion nanocomposite modified electrode. Microchim Acta 152:155
Zhou QX, Xiao JP, Wang WD, Liu GG, Shi QZ, Wang JH (2006) Determination of atrazine and simazine in environmental water samples using multiwalled carbon nanotubes as the adsorbents for preconcentration prior to high performance liquid chromatography with diode array detector. Talanta 68:1309
Li QL, Yuan DX (2004) Study of purified multi-walled carbon nanotubes as a sorbent of solid phase extraction for preconcentration of organophosphorous pesticides from water samples. J Xiamen University (Natural Science) 43:531
Basheer C, Alnedhary AA, Rao BSM, Valliyaveettil S, Lee HK (2006) Development and application of porous membrane-protected carbon nanotube micro-solid-phase extraction combined with gas chromatography/mass spectrometry. Anal Chem 78:2853
Zhou QX, Xiao JP, Wang WD (2007) Comparison of multiwalled carbon nanotubes and a conventional absorbent on the enrichment of sulfonylurea herbicides in water samples. Anal Sci 23:189
Zhou QX, Xiao JP, Wang WD (2006) Using multi-walled carbon nanotubes as solid phase extraction adsorbents to determine dichlorodiphenyltrichloroethane and its metabolites at trace level in water samples by high performance liquid chromatography with UV detection. J Chromatogr A 1125:152
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1.
Effect of eluent and its volume on recoveries of alachlor (n = 5). (DOC 23.5 KB)
Fig. S2.
Effect of eluent and its volume on recoveries of metolachlor (n = 5). (DOC 23.5 KB)
Fig. S3.
Effect of eluent and its volume on recoveries of butachlor (n = 5). (DOC 23.5 KB)
Fig. S4.
Effect of the volume of water samples on the recoveries. The RSDs were in the range of 4.69% ∼ 11.38% (n = 4). (DOC 25 KB)
Rights and permissions
About this article
Cite this article
Dong, M., Ma, Y., Zhao, E. et al. Using multiwalled carbon nanotubes as solid phase extraction adsorbents for determination of chloroacetanilide herbicides in water. Microchim Acta 165, 123–128 (2009). https://doi.org/10.1007/s00604-008-0109-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0109-z