Abstract
Purposes
Fine-needle aspiration cytology (FNAC) is a specific and important test used for the diagnosis of thyroid gland cancer. We developed a thyroid gland phantom using original manufacturing techniques and direct three-dimensional (3D) printing. The aim of this study was to confirm the effectiveness of this phantom by collecting data to evaluate puncture training.
Methods
Data from 45 ultrasonography-guided thyroid nodule FNAC procedures performed on our thyroid phantom were evaluated in our department. The first group comprised qualified physicians who specialized in thyroid gland treatment (group A; n = 10). The second and third groups comprised senior and junior residents (group B; n = 8 and group C; n = 12; respectively). The fourth group comprised students (group D; n = 15). We measured the times taken by these groups to complete each task.
Results
The skills of all participants in groups B, C, and D improved after using this phantom involving the major (parallel)- (0.47 ± 0.07) and short (orthogonal)-axes (0.52 ± 0.07) methods (P < 0.001). The number of erroneous punctures decreased from 53 to 3.
Conclusions
Our original phantom improved the puncture skills of students and junior doctors and was suitable as a tailored training model for practicing thyroid gland transfixion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The technical advances in recent decades have made many procedures, such as invasive examinations and surgeries, challenging; however, performing clinical procedures safely is crucial for both patients and clinicians. Fine-needle aspiration cytology (FNAC) is essential for the diagnosis of thyroid gland malignancy. When a lesion is detected in the thyroid gland, establishing if it requires surgery is important. Ultrasonography (US) is generally performed first, to determine whether further evaluation or FNAC is necessary.
Current clinical guidelines recommend FNAC for all solid nodules with a maximum diameter of > 2 cm on US, but it is not recommended for nodular lesions < 5 mm with no distant or lymph node metastases and low tumor marker levels [1]. FNAC is generally a safe examination [2, 3]; however, the procedure is made technically difficult by the proximity of the thyroid gland to the jugular veins, carotid arteries, and trachea. US-guided puncture, which is currently used for vascular access, drainage, and biopsy, can be performed during various steps of medical management, from diagnosis to treatment.
The advantages of FNAC include simplicity, minimal invasiveness, possible repetition, and high diagnostic accuracy, making it a widely used technique. Moreover, since imaging is obtained in real time, FNAC with US is useful for delineating the positional relationship between the target and the needle point for FNAC. However, obtaining safe, convenient, and precise access to complicated organ structures requires a high level of technical skill and expertise. Recent studies show a correlation between an increase in the rate of diagnosis of thyroid gland diseases and more training [4]. Acquiring these skills is essential for inexperienced doctors and students who have not yet decided on their area of specialization.
Within our facilities, phantom models are used to support education and training [5]. If phantoms tailored for individual patients are used for practice before clinical procedures are performed, the technique of FNAC can be mastered, which is important for medical trainees and inexperienced clinicians.
Reports have been published in this emerging field, describing fabricated phantoms of the breast [6], liver [7], uterus, and vagina [8]. However, to our knowledge, an elaborate phantom is not yet commercially available, and there have been no studies involving thyroid gland phantoms. Therefore, we constructed an elaborate phantom, taking size, touch, feel, and US views into consideration, using a hybrid of old and new manufacturing techniques, including direct three-dimensional (3D) printing and traditional mould-based manufacturing. The aim of this study was to evaluate the effectiveness of puncture training using our phantom for junior and senior residents and medical students.
Materials and methods
This study was approved by the Ethics Committee of Nagasaki University Hospital (Approval Number: 16042512). All participants provided written informed consent before participation.
Phantom fabrication procedure
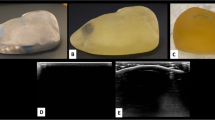
The procedure used to fabricate the thyroid gland phantom was described previously [9]. First, the computed tomography data were analyzed and 3D digital reconstruction was done, with output in an STL file format. Using our department’s 3D printer (Objet 260, Stratasys No. 33201; Eden Prairie, MN, USA), we created moulds for various organs, considering the limitation of the types of material that can be printed by a 3D printer. Currently available 3D printed materials are highly reflective of ultrasound waves; thus being non-permeable, preventing transparency. For the cervical vertebrae and trachea, the high reflectivity was acceptable, but it was not acceptable for other components, so a more traditional method of manufacturing was used. The 3D printer was used to create moulds for the thyroid gland, blood vessels, tumor, and surrounding soft tissue, which were made from ultrasound-permeable materials [9]. The moulds were injected with a chemical compound that confers a similar degree of permeability when viewed ultrasonically, as the organs they represent. The material was injected into the respective moulds of the blood vessels, the thyroid gland, and the tumor. Subsequently, the moulds were refrigerated at − 4 °C for approximately 15 min to harden. The objects were then removed from the moulds and colored, the components were positioned in the neck mould, and agar gel was poured into them. After approximately 2 h of refrigeration, the phantom was ready to be removed from the mould and used for US-guided puncture training. Two models, model 1 and model 2, were created with different tumor locations in the thyroid gland (Fig. 1A, B). Model 2 has a smaller target tumor and is positioned closer to the blood vessels than model 1.
Methods
We evaluated outcomes after allowing junior and senior residents and medical students to perform puncture training using this tailored thyroid gland phantom. We identified 45 US-guided thyroid nodule FNAC procedures performed with our thyroid phantom in our department between September 2015 and March 2016. Participants were classified into four groups. Group A comprised qualified physicians who specialized in breast and thyroid gland treatment (n = 10); Groups B and C comprised senior residents (n = 8) and junior residents (n = 12), respectively; and Group D comprised medical students (n = 15).
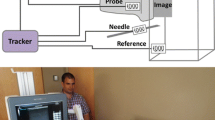
The parameters evaluated included the puncture time of the thyroid tumor, the preparation time (time needed to describe a lesion and prepare a transfixion), and the number of erroneous punctures. The procedures were measured using the major (parallel)- and short (orthogonal)-axes methods, and the same measurements were repeated after 1 month. An adapter was not used in either method during puncture. Evaluations were performed by group A using model 1. In contrast, participants in groups B, C, and D performed FNAC using a different phantom (model 2) for their first attempt (Fig. 2). Preparation time was defined as the time from placing the US probe on the phantom surface to placing the needle on the phantom surface. Puncture time was defined as the time taken to perform transfixion on the US screen and aspirate the blue liquid from inside the tumor.
Before the first puncture training, all members received the same fundamental information about thyroid diseases, neck dissection, and management confirmation of an echo probe. The practice method was repeated by participants in groups B, C, and D using model 1 after the first puncture, while all groups used model 2 for the first time in the second puncture. The goals of the practice were as follows:
-
1.
To delineate the tumor from blood vessels and identify the expected puncture location on the same screen.
-
2.
To firmly and continuously visualize the needle tip from the start of the puncture until the target was reached in the long axis method.
-
3.
To adjust the needle insertion angle, among other variables, so that the short axis method required as few punctures as possible.
If a participant failed to achieve each goal, they were instructed to review and understand the reasons for the failures; for example, not fixing the palm on the body surface, not moving the probe sideways during puncture, or inserting the needle in the wrong angle. The number of punctures differed, as the level of knowledge and skill varied; however, the maximum practice time was limited to 30 min. The participants were asked to complete a questionnaire after they finished their training.
Statistical analysis
The phantom was fabricated using a 3D printer (Objet 260, Stratasys, Eden Prairie, MN, USA) and puncture training was evaluated using a LOGIQ e Premium (GE Healthcare, Chalfont Saint Giles, UK) US diagnostic unit. The Student’s t test was used to compare average times between groups. Values of P < 0.05 were considered significant. MedCalc software version 11.5.1.0 (MedCalc Software, Mariakerke, Belgium) was used for statistical analysis.
Results
The preparation time increased based on the level of experience within the groups. However, the second puncture using the same phantom was significantly improved in group C (P < 0.05). Less preparation time was required for the short-axis method than for the major-axis method (Fig. 3A). Using models 1 and 2, significant improvements were observed in preparation time for all members of groups C and D (Fig. 3B). All participants in the four groups improved their puncture times by using the major- and short-axes methods for the same phantom (model 1) (Fig. 4A). When using the different phantoms (models 1 and 2), the puncture time did not change significantly in groups A and B, but it did improve significantly in groups C and D, to close to that in groups A and B (Fig. 5).
Preparation time. First attempt, model 1; second attempt, model 2. A Using the same model: first attempt, model 1; second attempt, model 1. The preparation time for the second attempt was shorter than that for the first attempt. A significant difference was observed in both methods among groups B, C, and D (P < 0.001). B When the other model was used: first attempt, model 1; second attempt, model 2
A Puncture time using model 1. Using the same phantom (model 1), reductions in time taken to perform the major-axis method were noted in all groups. A significant difference was observed in puncture times (P < 0.001). B Improvement rate for model 1. The improvement rate was defined to calculate the difference between the second attempt and the first attempt numerators and the first attempt denominator. The rate was then calculated by the time reduction ratio. The highest improvement rates were observed in group C, using the major-axis (0.58) and short-axis (0.62) methods
Puncture times using models 1 and 2. We evaluated the progress of all participants in groups B to D using phantom model 2. Using the major-axis method, a clear improvement was observed in groups B to D, contrary to group A. The greatest improvement was observed in group C. Using the shorts-axis method, the progress of all members in groups A to D was examined. The time shortening ratio was greater in groups B to D than in group A
The improvement rate was defined to identify the difference between the numerators for the first and second time practicing the procedure, and for the first time practicing the procedure. These values were measured using the ratio for time shortening. All participants in groups B, C, and D improved after using the major- (0.47 ± 0.07) and short- (0.52 ± 0.07) axes methods (P < 0.001). The highest improvement rate was observed in group C, using the major- (0.58) and short- (0.62) axes methods (Fig. 4B). After using a different phantom (model 2) during the first practice attempt, the progress of groups B to D was examined. Using the major-axis method, the mean time required for the second practice attempt (37.2 s) was longer than that required for the first practice attempt (24.5 s) for group A. Contrary to group A, significant improvements were observed in groups B, C, and D (group B, from 58.4 to 40.0 s; group C, from 160.4 to 45.6 s; and group D, from 163.3 to 69.7 s). Group C showed the most improvement. The progress of groups A to D in using the short-axis method was evaluated and the first average values of groups A, B, C, and D were 23.8, 53.8, 136.2, and 162.9 s, whereas the second average values were 20.3, 28.1, 34.5, and 45.3 s, respectively. The time shortening ratio was greater for groups B to D than for group A (Fig. 5). No significant difference was observed in preparation time or puncture time, and their relevance was not proven.
The numbers of erroneous punctures during the first practice attempt were 0, 2, 20, and 31 for groups A, B, C, and D, respectively. During the second attempt, a significant improvement was observed for the group D participants, who were three times better than with the first attempt (Table 1). The number of erroneous punctures was 31 (55%) when using the major-axis method. More vascular damage occurred when using the major-axis method, whereas more tracheal damage occurred when using the short-axis method (Table 2).
Discussion
Safety and accuracy are essential for all clinical procedures. FNAC is the most important diagnostic procedure used for outpatients with thyroid disease. However, achieving the required accuracy and safety can be difficult because of the complicated anatomy of the neck. Furthermore, teaching inexperienced doctors to perform FNAC in live patients is difficult because it is usually performed when the patients are awake. To address this issue, we created an original neck phantom with a thyroid tumor to train others to perform FNAC and then evaluated its effectiveness by assessing experienced and inexperienced doctors and medical students. Consequently, practicing FNAC on our phantoms helped inexperienced doctors and medical students improve the way they performed FNAC for thyroid tumors. Our data showed that practice improved the doctors’ skills, even when using the new model shape after practicing on the first model. Therefore, our neck phantom with similar US characteristics manufactured using patient data contributed to improving the FNAC competency of inexperienced doctors.
US has become the diagnostic imaging of choice for thyroid diseases because it is non-invasive and simpler than other diagnostic modalities [10]. According to an epidemiological survey, the incidence of thyroid disease is still high, with some thyroid anomaly found in 1 of 10 individuals [11]. In 2014, thyroid disease was diagnosed in approximately 86,700 patients [12]. Conversely, the number of thyroid disease specialists is insufficient despite the fact that thyroid gland morbidity has shown an upward trend in recent years [11]. Following a nuclear power accident in Japan, the importance of screening for thyroid nodules gained much attention. Therefore, more thyroid disease specialists are required and the training of these specialists is an urgent issue. To address this challenge, practicing in a laboratory using models based on patients can be helpful and effective.
The rate of detection of thyroid carcinoma using US is approximately 3.5-times higher than that using palpation [13]. Furthermore, FNAC is a simple, reliable, inexpensive, and generally safe diagnostic procedure for thyroid nodules. Local pain and minor hematomas are the most common post-FNAC complications, but severe complications are rare [2], although there are sporadic reports of severe complications after fine needle biopsy (FNB), such as uncontrolled hemorrhage or massive hematoma with airway obstruction [14,15,16,17], acute thyroid swelling after needle biopsy [18,19,20], infections [21], and tumor dissemination [22, 23]. While FNB is different from FNAC, it is challenging and associated with risk for inexperienced clinicians to perform independently because of the proximity of the thyroid to the jugular vein, carotid artery, and trachea. Moreover, US-guided puncture requires practice to achieve a certain level of skill before performing the procedure on a patient. In fact, in this study, errors were made during the procedure causing tracheal and vessel injury. In particular, resident doctors and medical students made many errors during practice; therefore, to perform their first procedure on a patient without any practice would be unacceptable. Our data showed that the number of errors during the procedure decreased dramatically after practice, indicating that our phantom could support improvements in the skill of inexperienced doctors and contribute ultimately to the safety and efficiency of this procedure.
The cost of developing a practice model is an important point to consider. Currently, 3D printers are popular in the medical field and are used to construct organ models and structures [15]. However, these printers are expensive and require a long period to create the model. To solve this issue, we used moulds to make some organs [9]. While 3D printers are needed to make the mould of the organ, after that, the cost of duplication is only about $5 (United States). Our phantoms are also elaborate because they are sonographically authentic and possess life-like elasticity, so should be considered suitable as practice models.
In our study, the puncture was performed using the major- and short-axes methods, which specialists must be adept at to perform the procedure successfully, even in patients with unusual anatomy or tumors. It has been reported that the short-axis approach takes less time than the major-axis approach when the catheter is inserted via the vessel in the neck. In our study, different types of puncture error were noted during the practice of the major and short-axes approaches. Therefore, mastering both methods will help reduce puncture errors. Our phantom is a useful tool for both methods because the US views are similar to the actual patient findings using both methods.
In conclusion, using a 3D printer, we successfully fabricated a tailored thyroid gland phantom representative of various patients, for doctors to practice transfixion. Our original phantoms improved the puncture skills of medical students and resident doctors effectively, resulting in their confidence and skill to ultimately perform safe and efficient procedures on patients.
References
Noboru H. Thyroid disease medical examination and treatment. Tokyo, Japan: Nankodo Co:p. 28–9; 2012
Meier CA. Thyroid nodules: pathogenesis, diagnosis and treatment. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(14):559–75. https://doi.org/10.1053/beem.2000.0103.
Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf). 2009;71:157–65. https://doi.org/10.1111/j.1365-2265.2009.03522.x.
Beland MD, Anderson TJ, Atalay MK, Grand DJ, Cronan JJ. Resident experience increases diagnostic rate of thyroid fine-needle aspiration biopsies. Acad Radiol. 2014;21:1490–4. https://doi.org/10.1016/j.acra.2014.06.006.
Takagi K, Nanashima A, Abo T, Arai J, Matsuo N, Fukuda T, et al. Three-dimensional printing model of liver for operative simulation in perihilar cholangiocarcinoma. Hepatogastroenterology. 2014;61:2315–6.
Gresens AA, Britt RC, Feliberti EC, Britt LD. Ultrasound-guided breast biopsy for surgical residents: evaluation of a phantom model. J Surg Educ. 2012;69:411–5. https://doi.org/10.1016/j.jsurg.2011.10.015.
Sugimoto K, Moriyasu F, Shiraishi J, Yamada M, Imai Y. A phantom study comparing ultrasound-guided liver tumor puncture using new real-time 3D ultrasound and conventional 2D ultrasound. AJR Am J Roentgenol. 2011;196:W753–7. https://doi.org/10.2214/AJR.10.5552.
Nattagh K, Siauw T, Pouliot J, Hsu IC, Cunha JA. A training phantom for ultrasound-guided needle insertion and suturing. Brachytherapy. 2014;13:413–9. https://doi.org/10.1016/j.brachy.2014.01.003.
Baba M, Matsumoto K, Yamasaki N, Shindo H, Yano H, Matsumoto M, et al. Development of a tailored thyroid gland phantom for fine-needle aspiration cytology by three-dimensional printing. J Surg Educ. 2017;74:1039–46. https://doi.org/10.1016/j.jsurg.2017.05.012.
The Japan Association of Breast and Thyroid Sonology. The guidebook of the thyroid ultrasound for diagnosis. Tokyo, Japan: Nankodo Co:p. 37; 2016
Noboru H. Thyroid disease medical examination and treatment. Tokyo, Japan: Nankodo Co:p. 3; 2012
The American Chamber of Commerce in Japan. Improvement of accuracy of breast cancer screening, lengthening healthy lifespans to boost economic growth, p 126–30; 2014
Shimura H. Characteristics and prevalence of thyroid incidentaloma. J Breast Thyroid Sonogr. 2016;5:5–9.
Noordzij JP, Goto MM. Airway compromise caused by hematoma after fine−needle aspiration. Am J Otolaryngol. 2005;26:398–9. https://doi.org/10.1016/j.amjoto.2005.02.016.
Roh JL. Intrathyroidal hemorrhage and acute upper airway obstruction after fine needle aspiration of the thyroid gland. Laryngoscope. 2006;116:154–6. https://doi.org/10.1097/01.mlg.0000187396.18016.d0.
Hor T, Lahiri SW. Bilateral thyroid hematomas after fine-needle aspiration causing acute airway obstruction. Thyroid. 2008;18:567–9. https://doi.org/10.1089/thy.2007.0363.
Ranta A, Salmi J, Sand J. Fatal cervical edema following diagnostic fine needle aspiration. Duodecim. 1996;112:2024–5.
Haas SN. Acute thyroid swelling after needle biopsy of the thyroid. N Engl J Med. 1982;307:1349. https://doi.org/10.1056/NEJM198211183072121.
Nakagawa Y, Hoshikawa S, Ozaki H, Mori K. Acute thyroid swelling after fine-needle aspiration biopsy. Int J Case Rep Imag. 2011;2:28–9. https://doi.org/10.5348/ijcri-2011-11-70-LE-8.
Van den Bruel A, Roelandt P, Drijkoningen M, Hudders JP, Decallonne B, Bouillon R. A thyroid thriller: acute transient and symmetric goiter after fine−needle aspiration of a solitary thyroid nodule. Thyroid. 2008;18:81–4. https://doi.org/10.1089/thy.2007.0118.
Chen HW, Tseng FY, Su DH, Chang YL, Chang TC. Secondary infection and ischemic necrosis after fine needle aspiration for a painful papillary thyroid carcinoma: a case report. Acta Cytol. 2006;50:217–20. https://doi.org/10.1159/000325936.
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Needle tract implantation of papillary thyroid carcinoma after fine-needle aspiration biopsy. World J Surg. 2005;29:1544–9. https://doi.org/10.1007/s00268-005-0086-x.
Shinohara S, Yamamoto E, Tanabe M, Maetani T, Kim T. Implantation metastasis of head and neck cancer after fine needle aspiration biopsy. Auris Nasus Larynx. 2001;28:377–80. https://doi.org/10.1016/s0385-8146(01)00093-1.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baba, M., Matsumoto, K., Shindo, H. et al. Development and evaluation of an original phantom model of ultrasonography-guided thyroid gland biopsy for the training of surgical residents and students. Surg Today 53, 443–450 (2023). https://doi.org/10.1007/s00595-022-02582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02582-9