Abstract
Purpose
The aim of this study was to evaluate anatomical resection (AR) versus non-AR for primary solitary hepatocellular carcinoma (HCC) with predicted microscopic vessel invasion (MVI) and/or microscopic intrahepatic metastasis (MIM).
Methods
This retrospective study included 358 patients who underwent hepatectomy and had no evidence of MVI and/or MIM on preoperative imaging. The predictors of MVI and/or MIM were identified. The AR group (n = 222) and the non-AR group (n = 136) were classified by number of risk factor, and the survival rates were compared.
Results
Microscopic vessel invasion and/or MIM were identified in 81 (22.6%) patients. A multivariate analysis showed that high des-gamma-carboxy prothrombin concentration [odds ratio (OR) 3.35], large tumor size (OR 3.16), and high aspartate aminotransferase concentration (OR 2.13) were significant predictors. The 5-year overall survival (OS) in the patients with zero, one, two, and three risk factors were 97.4%, 73.5%, 71.5%, and 65.5%, respectively. The OS of AR is superior to that of non-AR only in patients with one or two risk factors.
Conclusion
The present findings suggest that AR should be performed for patients with one or two risk factors, and that AR may prevent recurrence, as these patients are at risk of having MVI and/or MIM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer-related death in Japan [1]. Although the development of surgical techniques and perioperative management has achieved an overall survival (OS) rate of 40–60% at 5 years after hepatectomy, HCC is still difficult to cure due to its high recurrence rate [2,3,4]. Even when HCC is completely surgically removed, it often recurs due to microscopic intrahepatic metastasis (MIM) via microscopic vascular invasion (MVI), as HCC has a high propensity for invading intrahepatic vessels [5,6,7,8,9].
Anatomical resection (AR) is the complete excision of the tumor-bearing portal tributaries [10], and this procedure may prevent MIM by preventing the dissemination of hepatic parenchymal cells via the portal vein. A previous propensity score-matched study reported that AR is not superior to non-AR regarding the rates of local recurrence, OS, and relapse-free survival (RFS) in patients with solitary HCC [11].
However, AR may be superior to non-AR in patients with MVI, which is difficult to detect on preoperative imaging in the absence of macroscopic vascular invasion [12, 13]. Preoperative risk factors have previously been reported for the presence of MVI [14] and MIM [15]. The final goal of the present study was to identify the patients who would benefit from AR in terms of prognosis. We identified the risk factors for the presence of MVI and/or MIM in patients with a solitary HCC based on previous studies [14, 15], and compared the outcomes of AR versus non-AR in accordance with the number of risk factors for MVI and/or MIM.
Patients and methods
A total of 403 patients with a primary solitary HCC underwent hepatectomy with curative intent at the Division of Hepato-Biliary-Pancreatic Surgery, Shizuoka Cancer Center Hospital, between September 2002 and June 2017. We retrospectively reviewed the hospital database records until September 2019.
The present retrospective study was approved by the Institutional Review Board of Shizuoka Cancer Center (Approval Number: J2019-60) and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. Written informed consent was obtained for surgery and use of the patients’ clinical data.
All included patients had undergone computed tomography (CT) preoperatively. The methods used for CT imaging and judging the presence of macroscopic vessel invasion and/or intrahepatic metastasis have been described previously [14, 15]. The presence of macroscopic vessel invasion and/or intrahepatic metastasis was judged based on the radiology report.
All patients underwent preoperative blood examinations, such as viral serological testing, assessment of tumor markers and laboratory assessment of liver function. Liver function was assessed using the Child–Pugh classification [16]. The diagnosis of HCC was histopathologically confirmed in all patients. The typical part of the resected specimens was cut into serial 2–3 mm thick slices and fixed in 10% formalin for histopathological examinations. Based on the pathological report, MVI was defined as the presence of either microscopic portal vein invasion or venous invasion, while MIM was defined as the presence of microscopic intrahepatic metastatic tumors [14, 15]. The tumor stage was assessed on the basis of the 7th edition of the Union Internationale Contra le Cancer classification [17]. The details of the surgical strategy and procedure have been previously reported [11]. Briefly, AR was performed for HCC in patients with hepatitis B virus or no hepatitis infection if possible, whereas non-AR was performed in patients with hepatic C virus infection. The types of hepatectomies were defined in accordance with the Brisbane 2000 terminology [18]. In the present study, AR was defined as more than a resection of liver parenchyma dominated by the third-order subsegmental portal venous branches. In non-AR, liver resection was performed to secure a surgical margin of at least 5 mm, if possible; when this was impossible, liver resection was performed without exposing the tumor surface on the parenchymal transection.
Patients underwent physical examination and blood testing every 3 months postoperatively. Serial CT or liver ultrasonography was performed in each patient every 3–6 months. When HCC recurrence was detected, the most appropriate therapy was applied, after considering the patient’s liver function and tumor factors; therapy options included repeat hepatectomy, transcatheter arterial chemoembolization, radiofrequency ablation, or sorafenib.
Statistical analysis
Continuous variables are presented as the median and range and were compared using the Mann–Whitney U test. Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. All factors that were found to be significantly associated with the presence of MVI and/or MIM (P < 0.05) according to univariate analyses were entered into a multivariate analysis. The cut-off values of continuous variables were determined using receiver operating characteristic (ROC) curves and Youden’s index. The multivariate analysis was performed using the logistic regression method with a backward stepwise selection model. The survival period was defined as the time between the day of surgery and the event date (all-cause death for the OS, and recurrence for the RFS). The remaining patients were censored at the last follow-up visit in September 2019. The cumulative RFS and OS curves were analyzed using the Kaplan–Meier method, and were compared using the log-rank test. There were significant differences between the AR and non-AR group in liver-related factors, tumor-related factors, and etiology of liver disease. A propensity score matching (PSM) analysis was performed to remove any potential confounders which could lead to selection bias. All statistical analyses were performed using the SPSS 24.0 software program (SPSS, Inc., Chicago, IL, USA). Two-tailed P values of ≤ 0.05 were considered to be significant.
Results
Patient characteristics
Among the 403 patients with HCC, 44 were excluded from the present study due to the presence of macroscopic vessel invasion and/or intrahepatic metastasis on preoperative imaging, while one was excluded due to a lack of histopathological results. The remaining 358 patients were included in the present study. The patient characteristics are shown in Table 1. Among 358 patients, 222 patients (62.0%) underwent AR and there were 81 patients (22.6%) with MVI and/or MIM.
RFS and OS after AR versus non-AR in patients with MVI and/or MIM and those without MVI and/or MIM
The 5-year RFS and OS rates in the patients with MVI and/or MIM who underwent AR (23.4% and 52.3%, respectively) did not significantly differ from the rates in the patients with MVI and/or MIM who underwent non-AR (20.0% and 94.7%, P = 0.598 and P = 0.082, Fig. 1a, b, respectively). Furthermore, the 5-year RFS and OS rates in the patients without MVI and MIM who underwent AR (40.2% and 81.1%, respectively) did not significantly differ from the rates in the patients without MVI and MIM who underwent non-AR (42.3% and 73.6%, P = 0.543 and P = 0.067, Fig. 1c, d, respectively).
Relapse-free survival curve (a) and overall survival curve (b) after anatomical resection or non-anatomical resection in patients with hepatocellular carcinoma with microscopic vessel invasion and/or microscopic intrahepatic metastasis. The relapse-free survival curve (c) and overall survival curve (d) after anatomical resection or non-anatomical resection in patients with hepatocellular carcinoma without microscopic vessel invasion and/or microscopic intrahepatic metastasis
Uni- and multivariate analyses performed to identify the predictors of MVI and/or MIM in primary HCC
There were significant differences between the patients with and without MVI and/or MIM regarding four preoperative factors (Table 2). To convert these continuous variables to categorical variables, a ROC curve analysis was performed. In the multivariate analysis, the following factors remained significant independent preoperative predictors of MVI and/or MIM in patients with primary solitary HCC: des-gamma-carboxy prothrombin (DCP) > 51 mAU/mL, tumor diameter > 37 mm, and aspartate aminotransferase (AST) > 32 IU/L (Table 3).
RFS and OS in accordance with the number of risk factors for MVI and/or MIM
There were 48, 115, 114, and 81 patients with zero, one, two, and three of the identified risk factors, respectively. The incidences of MVI and/or MIM in the patients with zero, one, two, and three risk factors were 4.2%, 9.6%, 25.7%, and 48.1%, respectively, and the incidence of MVI and/or MIM significantly increased as the number of risk factors increased (P < 0.001).
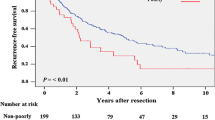
The 5-year RFS rates in the patients with zero, one, two, and three risk factors for MVI and/or MIM were 63.9%, 38.2%, 31.9%, and 26.9%, respectively (Fig. 2a). The RFS of the patients with zero risk factors was significantly better than that of the patients with one, two, and three risk factors (P = 0.011, P = 0.001, and P < 0.001, respectively). The RFS rate of the patients with three risk factors was significantly poorer than that of the patients with zero and one risk factor (P = 0.004 and P < 0.001, respectively). In contrast, the RFS rate did not significantly differ between patients with one versus two risk factors (P = 0.093), and between patients with two versus three risk factors (P = 0.119).
The 5-year OS in the patients with zero, one, two, and three risk factors for MVI and/or MIM were 97.4%, 73.5%, 71.5%, and 65.5%, respectively (Fig. 2b). The OS of the patients with zero risk factors was significantly better than that of the patients with one, two, and three risk factors (P = 0.001, P = 0.002, and P < 0.001, respectively). In contrast, the OS rate did not significantly differ among patients with one, two, and three risk factors.
AR versus non-AR classified by the number of risk factors for MVI and/or MIM
AR was performed in 18 (37.5%), 57 (49.6%), 77 (67.5%), and 70 (86.4%) patients with zero, one, two, and three risk factors for MVI and/or MIM, respectively. We divided the patients into three groups with zero, one or two, and three risk factors because the OS rates of the patients with one and two risk factors were almost the same, and there was a marginal difference in the OS of the patients with one or two risk factors versus those with three risk factors (P = 0.078). The RFS and OS of the patients who underwent AR and non-AR are shown in Fig. 3. The RFS did not significantly differ between the patients with zero (Fig. 3a, P = 0.288), one or two (Fig. 3b, P = 0.067), and three risk factors (Fig. 3c, P = 0.484). However, there was a marginal difference in the RFS of the patients with one or two risk factors. The OS did not significantly differ between the patients with zero (Fig. 3d, P = 0.583) and three risk factors (Fig. 3f, P = 0.462). The OS of AR was superior to that of non-AR only in the patients with one or two risk factors (Fig. 3e, P = 0.014).
Clinicopathological characteristics of the patients with one or two risk factors
Within the group of patients with one or two risk factors, there were significant differences between the AR and non-AR group in liver-related factors, tumor-related factors, and etiology of liver disease (Table 4). The one-to-one PSM selected 51 patients in each group. After PSM, there were no significant differences in RFS (Fig. 4a, P = 0.908) and clinicopathological characteristics between the AR and non-AR groups (Table 4). The OS of AR was still superior to that of non-AR after PSM (Fig. 4b, P = 0.034).
Discussion
The present study showed that the significant predictors of the presence of MVI and/or MIM in patients with primary solitary HCC were the preoperative DCP and AST levels, and tumor diameter, and that AR was useful for patients with one or two of these risk factors.
It remains controversial as to whether AR or non-AR is better for HCC. Some studies report that AR is oncologically superior to non-AR [10, 19,20,21,22,23,24,25,26,27], whereas others report that AR does not have superior outcomes to non-AR and thus recommend non-AR for HCC with cirrhosis to preserve liver function [11, 28,29,30,31,32]. A randomized controlled study is currently being performed to evaluate the impact of AR on the oncological outcomes of HCC (UMIN ID: C000000086). AR is technically challenging, especially in subsegmentectomy; however, it is important for surgeons to learn this procedure as one of the treatment options for HCC, regardless of the oncological benefit.
AR was first introduced in 1993 [10]. A study performed in 2005 showed that the OS and RFS of AR were superior to those of non-AR; however, the patient background characteristics significantly differed between the AR and non-AR groups, especially regarding liver function [19]. A study performed 10 years later showed that complete removal of the tumor-bearing portal territory, namely AR, decreases local tumor recurrence and improves RFS [26]. In contrast to the earlier study [19], this study showed that AR did not result in a superior OS compared with non-AR [26]. In 2015, a study published by a group of authors from the same department as the authors who performed the abovementioned studies [10, 19, 26] showed that the performance of three or more repeat hepatectomies has the same effect on the outcome as the performance of a second hepatectomy [33]. However, although the outcomes of AR and non-AR were equivalent, the authors emphasized the importance of performing AR in the first hepatectomy to obtain a good outcome after repeat hepatectomy, even if the repeat hepatectomy comprised non-AR [33]; this seems to contradict their opinion regarding the equivalence of AR and non-AR.
Overall, the findings of earlier studies related to AR seem to suggest that the outcome does not depend on the operative procedure (AR or non-AR), but rather depends on the treatment strategy of each facility [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Namely, it remains possible that the outcome after non-AR is poorer than that after AR because non-AR is only performed for patients with poor liver function in facilities where AR is performed to the extent allowed by liver function. It is possible that the outcome after non-AR is actually comparable to that after AR, as the non-AR group includes the patients with adequate liver function in facilities where the preferred surgical procedure is not AR.
In theory, the outcome of the patients without MVI and/or MIM should be equivalent after AR or non-AR, as the outcome depends on the liver function rather than on the operation procedure. In contrast, the outcome of patients with MVI and/or MIM should be improved by performing AR to completely remove the tumor-bearing portal territory. This theory is supported by a study in which AR significantly improved the RFS in patients with MVI [27], but is contradicted by a study in which AR for HCC with microscopic portal invasion (vp1) did not affect the RFS or OS [34]. This discrepancy between studies may be due to differences in the background viral status; the former study included numerous patients with the hepatitis B virus [27], while the latter study included numerous patients with the hepatitis C virus [34]. Patients with the hepatitis C virus often experience multi-centric recurrence. The present study also showed that AR did not affect the RFS or OS of patients with MVI and/or MIM in comparison with non-AR, similar to the outcomes of the latter study published by a Japanese group [34]. Although the present study showed that AR achieved a superior OS but not RFS in the patients with one or two risk factors, even after PSM, the concept of AR means that AR contributes to the improvement of RFS rather than OS. Although we have no clear explanation for this discrepancy, the present study included numerous patients with the hepatitis C virus (40.5%), so several recurrences in the non-AR group before PSM may have been multi-centric recurrences that could not be prevented by AR because the non-AR group had significantly poorer liver-related factors and a significantly higher rate of hepatitis C virus infection than the AR group. After PSM, the RFS curves of the AR and non-AR groups were similar because of the low incidence of multi-centric recurrence in the non-AR. However, it is difficult to determine the clear reason for the better OS in the AR group than the non-AR group in the present study. We therefore need to verify the reproducibility of the present results in an external validation set or in a prospectively study.
The present study identified three factors that were significant predictors of the presence of MVI and/or MIM in patients with primary solitary HCC, and showed that the outcome of patients with zero risk factors was significantly better than that of patients with risk factors, whereas the outcome of the patients with all three risk factors was worse than that of the patients with zero, one, and two risk factors. Among these patients, AR only affected the OS in patients with one or two risk factors. The present results suggest that AR is not needed in patients with none of these three risk factors, as the incidence of MVI and/or MIM in such patients is extremely low. In contrast, the outcome of patients with all three risk factors was substantially worse than that of patients with less than three risk factors, and even AR cannot prevent recurrence in patients with all three risk factors. This fact suggests that HCC may become systemic once the tumor has infiltrated the vasculature, and so AR might not prevent recurrence.
The present study is associated with several limitations. First, the retrospective single-center design may have resulted in selection bias. Therefore, we performed the PSM analysis to exclude the selection bias as much as possible. However, further prospective multi-institutional studies are needed to objectively validate the present results. Second, although the ORs slightly differed among the risk factors, the analyses were performed without considering the relative weight or importance of each of the three predictors for the presence of MVI and/or MIM. Finally, the incidence of MVI and/or MIM in the patients with one or two risk factors whose prognosis were improved by AR was quite low (17.5%). The possible reason for the low incidence of MVI and/or MIM in the current study depended on the fact that not all of the specimens were cut into the slide, and then we might miss the presence of MVI and/or MIM in some patients. Such patients therefore may have higher incidence of MVI and/or MIM than that in the current value if all the specimens were cut into the slides.
In conclusion, the present findings suggest that AR should be performed for patients with one or two risk factors for the presence of MVI and/or MIM, and that AR may prevent recurrence because these patients have a certain risk of MVI and/or MIM.
References
Vital statistics, Ministry of Health, Labour and Welfare: Available from: http://www.mhlw.go.jp/english/ database/db-hw/vs01.html Cited 25 September 2017.
M Kudo N Izumi M Sakamoto Y Matsuyama T Ichida O Nakashima et al Liver Cancer Study Group of Japan. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5:190–7.
Shim JH, Jun MJ, Han S, Lee YJ, Kim KM, Lim YS, Lee HC. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261:939–46.
Chapman WC, Klintmalm G, Hemming A, Vachharajani N, Majella Doyle MB, DeMatteo R, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. 2015;220:628–37.
Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6:259–66.
Yamamoto T, Kajino K, Kudo M, Sasaki Y, Arakawa Y, Hino O. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology. 1999;29:1446–52.
Yasui M, Harada A, Nonami T, Takeuchi Y, Taniguchi K, Nakao A, Takagi H. Potentially multicentric hepatocellular carcinoma: clinicopathologic characteristics and postoperative prognosis. World J Surg. 1997;21:860–4.
Matsuda M, Fujii H, Kono H, Matsumoto Y. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353–9.
Arii S, Monden K, Niwano M, Furutani M, Mori A, Mizumoto M, Imamura M. Results of surgical treatment for recurrent hepatocellular carcinoma; comparison of outcome among patients with multicentric carcinogenesis, intrahepatic metastasis, and extrahepatic recurrence. J Hepatobiliary Pancreat Surg. 1998;5:86–92.
Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304.
Okamura Y, Ito T, Sugiura T, Mori K, Uesaka K. Anatomic versus nonanatomic hepatectomy for a solitary hepatocellular carcinoma : a case-controlled study with propensity score matching. J Gastrointest Surg. 2014;18:1994–2002.
Cucchetti A, Piscaglia F, Frigioni AD, Ravaioli M, Cescon M, Zanello M, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and microvascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880–8.
Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44:846–53.
Okamura Y, Sugiura T, Ito T, Yamamoto Y, Ashida R, Aramaki T, et al. The predictors of microscopic vessel invasion differ between primary hepatocellular carcinoma and hepatocellular carcinoma with a treatment history. World J Surg. 2018;42:3694–704.
Okamura Y, Sugiura T, Ito T, Yamamoto Y, Ashida R, Aramaki T, et al. The tumor diameter cut-off for predicting microscopic intrahepatic metastasis of hepatocellular carcinoma patients without treatment history differs from that of hepatocellular carcinoma patients with a treatment history. Clin Transl Oncol. 2020;22:319–29.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the esophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9.
Sobin LH, Gospodarowicz MK, Wittekind CH, editors. TNM classification of malignant tumours. 7th ed. New York: Wiley-Liss; 2009.
Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–5.
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–9.
Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma: a univariate and multivariate analyses. Hepatology. 1991;14:802–5.
Izumi R, Shimizu K, Ii T, Muraoka K, Inoue T, Fukushima W, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–7.
Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, Blumgart L, Brennan M. Factors affecting long-term outcomeafter hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28–35.
Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219–22.
Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, et al. Significance of anatomic resection for early and advanced hepatocellular carcinoma. Langenbecks Arch Surg. 2012;397:85–92.
Wakai T, Shirai Y, Sakata J, Kaneko K, Cruz PV, Akazawa K, et al. Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1356–65.
Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64:594–600.
Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, Qiu Y. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017;32:870–8.
Yamashita Y, Taketomi A, Itoh S, Kitagawa D, Kayashima H, Harimoto N, et al. Longterm favorable results of limited hepatic resections for patients with hepatocellular carcinoma: 20 years of experience. J Am Coll Surg. 2007;205:19–26.
Kaibori M, Matsui Y, Hijikawa T, Uchida Y, Kwon AH, Kamiyama Y. Comparison of limited and anatomic hepatic resection for hepatocellular carcinoma with hepatitis C. Surgery. 2006;139:385–94.
Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, Togo S. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008;143:607–15.
Marubashi S, Gotoh K, Akita H, Takahashi H, Ito Y, Yano M, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102:776–84.
Li SQ, Huang T, Shen SL, Hua YP, Hu WJ, Kuang M, et al. Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. Br J Surg. 2017;104:118–27.
Mise Y, Hasegawa K, Shindoh J, Ishizawa T, Aoki T, Sakamoto Y, et al. The feasibility of third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg. 2015;262:347–57.
Hidaka M, Eguchi S, Okuda K, Beppu T, Shirabe K, Kondo K, et al. Impact of anatomical resection for hepatocellular carcinoma with microportal invasion (vp1): a multi-institutional study by the Kyushu Study Group of Liver Surgery. Ann Surg. 2020;271:339–46.
Acknowledgments
We thank Kelly Zammit, BVSc, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest regarding the current study.
Ethical approval
This study confirmed to the ethical guidelines of the Declaration of Helsinki (2013 revision) and was retrospective in nature, and we obtained approval from the Institutional Review Board of Shizuoka Cancer Center for the exception of patient consent (Approval Number: J2019-60).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okamura, Y., Sugiura, T., Ito, T. et al. Anatomical resection is useful for the treatment of primary solitary hepatocellular carcinoma with predicted microscopic vessel invasion and/or intrahepatic metastasis. Surg Today 51, 1429–1439 (2021). https://doi.org/10.1007/s00595-021-02237-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02237-1