Abstract
The Kono-S anastomosis was introduced in 2011 as an alternative anastomosis in Crohn’s disease (CD) surgery. Since then, prevailing evidence of the favorable results of the Kono-S anastomosis has been published from around the world. We conducted this study to analyze the effectiveness of the Kono-S anastomosis, by searching Medline, Embase, CNKI, and google scholar. Binominal data were analyzed after Freeman–Tukey double-arcsine transformation. Comparative data were analyzed using the Mantel–Haenszel model for dichotomous outcomes and the mean difference for continuous outcomes. We identified 676 patients who underwent surgery with a Kono-S anastomosis. Surgical recurrence was pooled at an average of 0% (CI: 0.00–0.01) and a reduced mean Rutgeerts score of 1.375 (CI: 0.727–2.023) after Kono-S anastomosis. Endoscopic recurrence after sensitivity analysis was 5% (CI: 0.00–0.15). Complications were rare, with a 3% incidence of ileus (CI: 0.01–0.05), a 4% incidence of small bowel obstruction (CI: 0.01–0.10), a 1% incidence of an anastomotic leak incidence (CI: 0.00–0.03), and a 10% incidence of postoperative infection (CI: 0.03–0.20). Evidence from this meta-analysis favors the Kono-S anastomosis for CD patients, especially for ileocolic anastomosis. Thus, clinicians should consider the applicability of Kono-S anastomosis in respective institutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is characterized by transmural inflammation of the bowel and can result in strictures, fistulas, and abscess [1]. Despite remarkable improvements in immunotherapy for CD, the percentage of CD patients who require surgery remains high, at 74–80% [1, 2]. Although recent biologic therapies have reduced the rates of surgery in the short term, they are still unable to effectively reduce the eventual need for surgery [2, 3].
In the 1990s, postoperative surgical and endoscopic recurrence at 12 months was as high as 90% [4, 5]. While the rate of surgery has decreased, surgical anastomoses remain one of the mainstream treatments of CD. Current methods of reducing recurrence rates include postoperative prophylaxis with biologics, novel surgical techniques, or reducing patient exposure to risk factors [6]. In particular, the type of anastomosis has been a subject of debate, with the more popular conventional methods of anastomosis being handsewn end-to-end anastomosis (HEEA) or stapled side-to-side anastomosis (SSSA). The stapled side-to-side anastomosis technique is thought to be superior in terms of postsurgical recurrence [6]; however, many of these CD patients eventually need reoperation to address disease recurrence in these anastomotic sites [1].
With the aim of reducing anastomotic recurrences, Kono developed a new antimesenteric functional end-to-end handsewn anastomosis: the “Kono-S anastomosis” [1]. This procedure involves transecting the bowel with a linear cutter and suturing both stumps together to create a supporting column, followed by a functional end-to-end handsewn anastomosis [1]. Preliminary studies comparing the Kono-S anastomosis with other surgical techniques (HEEA or SSSA) have shown promise, with the Kono-S method proving successful in reducing the endoscopic recurrence score (mean Rutgeerts score 2.6 vs 3.4; P = 0.008) and surgical recurrence rates (0% vs 15%; P < 0.0013) of CD patients [1]. Hence, we conducted a meta-analysis to assess the clinical outcomes of Kono-S surgery and evaluate its overall efficacy and safety.
Methods
The PRISMA guidelines were used in the synthesis of the review [7]. A search strategy was conducted on May 12, 2020, using Medline, Embase, CNKI, and clinicaltrials.gov, to identify articles relating to Kono-S anastomosis in CD surgery. Additional searches were carried out via Google Scholar by sieving through articles for citations of Kono et al. [1]. All citations were managed by EndNote X9. The sieving of articles was undertaken based on a pre-specified inclusion criterion. Both comparative and non-comparative studies were included in the analysis. Finalized included articles were systematically extracted with a structured proforma including information about population demographics (age, gender, previous bowel surgery, perioperative medications, postoperative medications, smokers), clinical outcomes (endoscopic, surgical, and clinical recurrence), and complications arising from Kono-S anastomoses. Endoscopic recurrence was defined by the Rutgeerts endoscopic score. When the mean and standard deviation were unavailable, existing formulas were used in the conversion [8].
Single-arm meta-analysis was conducted for binominal and continuous data. A meta-analysis of binominal data was done after a Freeman–Tukey double-arcsine transformation, and effect size was pooled with random effects by DerSimonian and Laird [9]. A meta-analysis of comparisons was done with the Mantel–Haenszel model for dichotomous outcomes and the mean difference (MD) for continuous outcomes [10]. When possible, meta-regression was used to explore the heterogenicity and relationship of covariates with the outcomes. A random-effects meta-regression with residual maximum likelihood and the Knapp and Hartung variance estimator was used for the analysis [11, 12]. All analyses were conducted in STATA 16.1 and RevMan 5.3.
Results
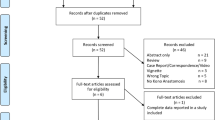
Our search strategy identified 223 articles, from among which 15 underwent full text analysis. Articles were excluded if they were a duplicated study, review, commentary, or were unspecific towards Kono-S anastomosis. Nine articles were included in the final review, with the remainder being general HEEA articles. Three of these articles originated from Japan [1, 13, 14], two from Italy [15, 16], two from the USA [17, 18], one from Germany [19], and one was a multicenter study in Japan and the USA [20]. A total of 676 patients underwent Kono-S surgery for CD largely originating from the ileocolic region. The mean age of the patients ranged from 33.5 to 39 years old across the included studies. The majority (n = 8) of studies were observational in nature (retrospective and prospective cohorts), and there was one randomized controlled trial [15]. Figure 1 shows a flowchart of the systematic review. Table 1 summarizes the included articles on Kono-S anastomosis, and Supplementary Table 1 summarizes those on conventional anastomosis.
Clinical Outcomes
The rates of surgical recurrence, endoscopic recurrence, mean Rutgeerts score, and 5 year surgery-free recurrence were the main outcomes in the meta-analysis. Of the 607 Kono-S patients, 531 were evaluated for surgical recurrence, which was reported with a pooled average incidence of 0% (6/531, CI: 0.00–0.01, Fig. 2). The mean follow-up duration ranged from 12.75 months to 77.75 months. Meta-regression analysis found no significant difference in follow-up durations (β = 0.0002, SE = 0.0002, p = 0.375). The 5 year surgery-free recurrence rate was reported as 97% (253/261, CI: 0.95–1.00). The cumulative incidence of endoscopic recurrence was 15% (63/168, CI: 0.00–0.47). The mean follow-up duration ranged from 6.4 months to 18 months. When sensitivity analysis was performed to exclude the first attempt by Kono et al., the cumulative incidence of endoscopic recurrence was significantly reduced to 5% (14/109, CI: 0.00–0.15). Severe endoscopic recurrence (Rutgeerts score ≥ 3) was reported with a 6% incidence (7/73, CI: 0.01–0.14). Clinical recurrence (Crohn’s disease activity index (CDAI) > 200) was reported only by Luglio et al. at 12 and 24 months, where Kono-S anastomosis was superior to stapled ileocolic side-to-side anastomosis (8% vs 18%, P = 0.2 and 11.1% vs 30.2%, P = 0.04).
The pooled mean Rutgeerts score was 1.153 (n = 437, CI: 0.553–1.754) with a mean follow-up duration ranging from 6.4 months to 60 months. However, this score was affected by follow-up duration in the meta-regression analysis (β = 0.035, SE = 0.006 p = 0.024), with a longer follow-up time being associated with a significant increase in the mean Rutgeerts score. When the mean Rutgeerts score was regressed with the proportion of ileocolic anastomosis, it was significant (β = − 4.095, SE = 1.359 p = 0.030). Thus, the reduction in the Rutgeerts score was more pronounced in studies with a higher proportion of ileocolic anastomosis.
When Kono-S anastomosis was compared with conventional anastomoses, the Kono-S anastomosis resulted in a significant decrease in the odds of surgical recurrence (n = 436, OR: 0.10, CI: 0.04–0.26, p < 0.00001, Fig. 3). The 5 year surgery-free recurrence was also significantly higher than that of the controls (n = 357, OR: 8.62, CI: 0.97 to 76.36, p = 0.050), but endoscopic recurrence was not significantly different between the Kono-S and conventional anastomoses (OR: 0.46 CI: 0.06–3.45, p = 0.450). The mean difference in the Rutgeerts score was significantly reduced after Kono-S vs conventional anastomosis (n = 221, MD: − 0.90 CI: − 1.48 to − 0.32, p = 0.002).
Surgical Outcomes
The average length of hospital stay was 7–15 days. The duration of surgery was pooled at 179.085 min (n = 236, CI: 122.619 min–235.552 min), and the duration of surgery was not significantly different from that of surgery with conventional anastomosis (n = 379, MD: − 1.09 min, CI: − 18.16mins to 15.98mins, p = 0.900). Blood loss was reported in two papers (median = 239, IQR: 105–450 and mean = 153, range 5–1,100), and the pooled incidence of blood transfusion was 11% (16/147 CI: 0.06–0.16) [13, 14]. In the report by Luglio et al., the average time to flatus or stool was 3 ± 1 day.
Complications
In general, complications were rare in Kono-S surgery. No mortality resulting from surgery was reported in any of the included articles. The pooled incidence of ileus was 3% (10/319, CI: 0.01–0.05), and small bowel obstruction was reported as 4% (18/334, CI: 0.01–0.10). The cumulative anastomotic leak incidence was 1% (11/525, CI: 0.00–0.03; Fig. 4), and the incidence of anastomotic bleeding was 1% (1/105, CI: 0.00–0.04). There was an increased incidence of other postoperative complications such as superficial and deep organ infections (51/436, 10% CI: 0.03–0.20 and 21/370, 5% CI: 0.02–0.09 respectively). When Kono-S was compared with conventional methods, there was no significant difference in the occurrence of ileus and anastomotic bleeding (n = 221, OR: 1.13, CI: 0.16–8.16, p = 0.120 and n = 221, OR: 0.44, CI: 0.06–3.38, p = 0.43 respectively). There was also a lower incidence of anastomotic leak in the Kono-S patients than in the controls (n = 436, OR: 0.24, CI: 0.10–0.61, p = 0.003). Infection rates were comparable between the Kono-S patients and the controls, without a significant difference (n = 436, OR: 1.13, CI: 0.66–1.94, p = 0.667). Kono-S anastomosis resulted in fewer anastomotic fistulae than side-to-side anastomosis (0% vs 2.3%).
Discussion
This meta-analysis summarizes the evidence from studies on the Kono-S anastomosis in CD surgery. Pooled evidence across the studies shows the promise of reduced surgical and endoscopic recurrence. In the era of ever-advancing biologics, the utility of surgery is being questioned in the treatment of CD. However, historical estimates suggest that up to 70% of patients will undergo surgery, which will remain a mainstream option despite the advances in biologic treatment [21, 22]. A recent randomized controlled trial comparing the use of biologics vs early surgery for ileocolic CD showed better quality of life (QOL) measures and cost-effectiveness with surgery [23]. Some observational studies also found that patients had a rate of surgery of 7–24.9%, despite being on biologics [24]. Moreover, the cost of biological therapy can be prohibitive in the potential applicability of biologics use [15, 25]. Thus, surgery remains one of the most affordable and effective treatments of CD.
Traditional HEEA or SSSA is the conventional anastomosis used in CD surgery. While SSSA is superior to HEEA in terms of short-term postoperative complications, both techniques have similar long-term CD recurrence [25]. In 2011, Kono et al. proposed a novel method of end-to-end anastomosis with less surgical recurrence than conventional anastomotic techniques [1]. The defining feature of Kono et al.’s surgery is the creation of a supporting column, sited posteriorly on the mesenteric border, which is proposed by Kono to be the most common site of recurrence. This posterior column prevents the distortion and subsequent stenosis of the anastomosis (Fig. 5). It is also thought that the anastomosis allows for better healing with the preservation of innervations and blood supply. The last benefit of Kono-S anastomosis is that the isoperistaltic orientation allows for postoperative endoscopy.
Kono-S anastomosis has been adopted by countries beyond Japan, including Italy, the USA, and Germany, with an additional ongoing trial to evaluate its clinical applicability (NCT03256240). Clearly, the reproducibility of Kono-S anastomosis has been demonstrated, with further evidence substantiating its use from the SuPREMe-CD trial [15]. When incidence was pooled from Kono-S only, surgical recurrence across 531 patients was 0% (CI: 0.00–0.01). However, surgical recurrence was defined clearly by only three articles as reoperation at the anastomotic site [1, 13, 20] and by Luglio et al. as re-operation in the presence of symptoms [15], with the remainder of the articles not having a clear definition. While the reported rates are low, this lack of clarity can potentially confound the results. The incidence of endoscopic recurrence was 15% (63/168, CI: 0.00–0.47), which dropped to 5% (14/109, CI: 0.00–0.15) with the exclusion of the Kono-S original study [1]. This first Kono-S study observed a higher rate of endoscopic recurrence (12 months, 49/59; 83%) than other studies (2–25%), possibly because the procedure was in its infancy and there was an associated learning curve. Thus, a more conservative estimate of endoscopic recurrence with Kono-S anastomosis is 5% (14/109, CI: 0.00–0.15). When comparing endoscopic recurrence between Kono-S and conventional anastomosis, significance was not achieved, probably because of the increased rates from the original Kono et al. study [1]. The maturation of further studies is required to investigate if the Kono-S anastomosis achieves superior rates of endoscopic recurrence.
In this review, 21–100% of patients underwent endoscopic examination. The pooled mean Rutgeerts score was also noted to be low at 1.153 (n = 437, CI: 0.553–1.754), but was marginally affected by the follow-up duration in regression analysis (β = 0.035, SE = 0.006 p = 0.024). In our meta-regression, studies with an increased proportion of ileocolic anastomosis had a strong association with a lower Rutgeerts score on follow-up (β = − 4.095, SE = 1.359 p = 0.030). Since 55% of cases of CD affect the ileocolic region, it is the most common location for resections [26], and this finding supports the use of Kono-S anastomosis, especially for ileocolic anastomoses. We have a hypothesis for this difference. Beyond the supporting column, Kono-S anastomosis is also an isoperistaltic anastomosis, which is configured to be larger than the conventional HEEA. The loss of the ileocecal valve potentially results in changes to ileal gut flora from cecal–ileal reflux [27, 28]. Kono-S anastomosis might bring about the “best of both worlds” of conventional anastomoses, resulting in less anastomotic disruption and fewer flora changes. As we know, the profound impact of gut flora on the prognosis and management of CD is evolving rapidly with changes in biodiversity affecting relapse and the isoperistaltic nature, along with the supporting column in Kono-S, which might diminish the effect of flora change [29, 30]. Future prospective studies are needed to validate the impact and mechanism of different anastomoses on CD recurrence.
In our meta-analysis, Kono-S anastomosis resulted in a significantly lower odds (n = 436, OR: 0.10, CI:0.04–0.26, p < 0.001) of surgical recurrence and a lower Rutgeerts score than conventional anastomosis (MD: − 0.90 CI: − 1.48 to − 0.32, p = 0.002). The cumulative 5 year surgical free recurrence rate was reported at 98% (322/330, CI: 0.95–1.00), with 8.62 times (n = 357, CI: 0.97–76.36, p = 0.050) increased odds of surgical-free recurrence vs conventional anastomosis. Comparatively, evidence from a randomized controlled trial between HEEA and SSSA shows 5 year reoperation rates of 25% and 8%, respectively [31]. While still a relatively new procedure, evidence of the advantages of the Kono-S procedure is seen in the reduced surgical reoperation rate at 5 years. Complications rates were also relatively low after Kono-S anastomosis, at an estimated 4% (18/334, CI: 0.01–0.10) for small bowel obstruction, 3% (CI: 0.01–0.05) for ileus, 1% (CI: 0.00–0.03) for anastomotic leak, and 1% (1/105, CI: 0.00–0.04) for anastomotic bleeding. Infections were the most common complication, reported in only 8% (53/505, CI: 0.03–0.17) of patients. Compared with conventional anastomoses, most complications were not significant, aside from the decreased odds of anastomotic leak with Kono-S (n = 436, OR: 0.24, CI: 0.10–0.61, p = 0.003).
The influence of postoperative medical prophylaxis on the recurrence of CD must be taken into consideration. In our review, 28–63% of patients were on postoperative biologics. It is also unclear from the studies which criteria were used to decide if patients needed postoperative medical prophylaxis, the surveillance protocol, and the threshold for step-up to biologics. The rate of surgery in patients using biologics is estimated to be around 9.2–17.2% at 24 months; thus, more studies are required to evaluate the influence of biologics on recurrence after Kono-S anastomosis [24, 32,33,34]. Biologics can be limited in their application, primarily due to cost constraints and an estimated one-third of patients failing to respond [35]. A Kono et al. subgroup analysis of infliximab use found that surgical recurrence was unaffected by the use of biologics. Although this observation is yet to be replicated in other studies, preliminary evidence suggests that Kono-S anastomosis is potentially useful for patients who are unsuitable for biologic therapy, either because of cost constraints or failure of therapy. Beyond surgical recurrence, biologics are still required for the prevention of clinical and endoscopic recurrence [34].
Conclusion
The Kono-S anastomosis in CD appears to have low complication and recurrence rates, mainly for ileocolic anastomosis. Since its introduction, the Kono-S anastomosis has been used in countries around the world, with evidence of reproducibility. However, more studies are needed to investigate the mechanism and clinical impact of Kono-S anastomosis on CD recurrence.
References
Kono T, Ashida T, Ebisawa Y, Chisato N, Okamoto K, Katsuno H, et al. A new antimesenteric functional end-to-end handsewn anastomosis: surgical prevention of anastomotic recurrence in Crohn's disease. Dis Colon Rectum. 2011;54(5):586–92. https://doi.org/10.1007/DCR.0b013e318208b90f.
Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn's disease. Br J Surg. 2000;87(12):1697–701. https://doi.org/10.1046/j.1365-2168.2000.01589.x.
Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54(2):237–41. https://doi.org/10.1136/gut.2004.045294.
Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136(2):441–450.e441. https://doi.org/10.1053/j.gastro.2008.10.051.
Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99(4):956–63. https://doi.org/10.1016/0016-5085(90)90613-6.
Kano M, Hanari N, Gunji H, Hayano K, Hayashi H, Matsubara H. Is "functional end-to-end anastomosis" really functional? a review of the literature on stapled anastomosis using linear staplers. Surg Today. 2017;47(1):1–7. https://doi.org/10.1007/s00595-016-1321-9.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097–e10000971000097. https://doi.org/10.1371/journal.pmed.1000097.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. https://doi.org/10.1186/1471-2288-14-135.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72(1):39. https://doi.org/10.1186/2049-3258-72-39.
Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14(1):30. https://doi.org/10.1186/1471-2288-14-30.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–710. https://doi.org/10.1002/sim.1482.
Harbord RM, Higgins JPT. Meta-regression in stata the stata journal. 2008;8(4):493–519. https://doi.org/10.1177/1536867X0800800403.
Shimada N, Ohge H, Kono T, Sugitani A, Yano R, Watadani Y, et al. Surgical recurrence at anastomotic site after bowel resection in Crohn's disease: comparison of Kono-S and end-to-end anastomosis. J Gastrointest Surg. 2019;23(2):312–9. https://doi.org/10.1007/s11605-018-4012-6.
Katsuno H, Maeda K, Hanai T, Masumori K, Koide Y, Kono T. Novel antimesenteric functional end-to-end handsewn (Kono-S) anastomoses for Crohn's disease: a report of surgical procedure and short-term outcomes. Dig Surg. 2015;32(1):39–44. https://doi.org/10.1159/000371857.
Luglio G, Rispo A, Imperatore N, Giglio MC, Amendola A, Tropeano FP, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn's disease: the SuPREMe-CD study–a randomized clinical trial. Ann Surg. 2020. https://doi.org/10.1097/sla.0000000000003821.
Fichera A, Zoccali M, Kono T. Antimesenteric functional end-to-end handsewn (Kono-S) anastomosis. J Gastrointest Surg. 2012;16(7):1412–6. https://doi.org/10.1007/s11605-012-1905-7.
Lin D, Krane M, Umanskiy K, Rubin M, Rubin D, Hurst R, et al. Surgical prophylaxis of Crohn's disease recurrence: the Kono-S anastomosis. Preliminary results of a single-institution experience: P-52. Inflamm Bowel Dis. 2011;17(Suppl_2):S27–S2727. https://doi.org/10.1097/00054725-201112002-00089.
Krane MK, Cannon LM, Allaix Marco E, Kono T, Fichera A. A new antimesenteric functional end-to-end handsewn (Kono-S) anastomosis: feasibility and short-term outcomes in Crohn’s disease. J Am Coll Surg. 2015;221(4, Supplement 2):e5. https://doi.org/10.1016/j.jamcollsurg.2015.08.313.
Seyfried S, Post S, Kienle P, Galata CL. Die Kono-S-anastomose in der Chirurgie des Morbus Crohn. Der Chirurg. 2019;90(2):131–6. https://doi.org/10.1007/s00104-018-0668-4.
Kono T, Fichera A, Maeda K, Sakai Y, Ohge H, Krane M, et al. Kono-S Anastomosis for surgical Prophylaxis of anastomotic recurrence in Crohn's disease: an international multicenter study. J Gastrointest Surg. 2016;20(4):783–90. https://doi.org/10.1007/s11605-015-3061-3.
Frolkis AD, Dykeman J, Negron ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006.
Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn's disease: what is the actual risk? Gut. 2011;60(9):1178–81.
Ponsioen CY, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Bossuyt PMM, Hart A, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2(11):785–92.
Wong DJ, Roth EM, Feuerstein JD, Poylin VY. Surgery in the age of biologics. Gastroenterol Rep (Oxf). 2019;7(2):77–90.
Patel KV, Darakhshan AA, Griffin N, Williams AB, Sanderson JD, Irving PM. Patient optimization for surgery relating to Crohn's disease. Nat Rev Gastroenterol Hepatol. 2016;13(12):707–19. https://doi.org/10.1038/nrgastro.2016.158.
Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380(9853):1590–605. https://doi.org/10.1016/s0140-6736(12)60026-9.
Machado WM, Miranda JRA, Morceli J, Padovani CR. The small bowel flora in individuals with cecoileal reflux. Arq Gastroenterol. 2008;45:212–8.
Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113(5):1767–78. https://doi.org/10.1053/gast.1997.v113.pm9352883.
Magro DO, Santos A, Guadagnini D, de Godoy FM, Silva SHM, Lemos WJF, et al. Remission in Crohn’s disease is accompanied by alterations in the gut microbiota and mucins production. Sci Rep. 2019;9(1):13263. https://doi.org/10.1038/s41598-019-49893-5.
Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An overview of the bacterial contribution to Crohn disease pathogenesis. J Med Microbiol. 2016;65(10):1049–59. https://doi.org/10.1099/jmm.0.000331.
Ikeuchi H, Kusunoki M, Yamamura T. Long-term results of stapled and hand-sewn anastomoses in patients with Crohn's disease. Dig Surg. 2000;17(5):493–6. https://doi.org/10.1159/000051946.
Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. Am J Gastroenterol. 2013;108(11):1731–42. https://doi.org/10.1038/ajg.2013.287.
Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn's disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18(9):1617–23. https://doi.org/10.1002/ibd.21928.
De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385(9976):1406–17. https://doi.org/10.1016/S0140-6736(14)61908-5.
Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn's disease. Therap Adv Gastroenterol. 2010;3(3):179–89. https://doi.org/10.1177/1756283X09357579.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
None of the authors has any conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ng, C.H., Chin, Y.H., Lin, S.Y. et al. Kono-S anastomosis for Crohn’s disease: a systemic review, meta-analysis, and meta-regression. Surg Today 51, 493–501 (2021). https://doi.org/10.1007/s00595-020-02130-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02130-3