Abstract
Organ liver transplantation and hepatocyte transplantation are not performed to their full potential because of donor shortage, which could be resolved by identifying new donor sources for the development of hepatocyte-like cells (HLCs). HLCs have been differentiated from some stem cell sources as alternative primary hepatocytes throughout the world; however, the currently available techniques cannot differentiate HLCs to the level of normal adult primary hepatocytes. The outstanding questions are as follows: which stem cells are the best cell sources? which protocol is the best way to differentiate them into HLCs? what is the definition of differentiated HLCs? how can we enforce the function of HLCs? what is the difference between HLCs and primary hepatocytes? what are the problems with HLC transplantation? This review summarizes the current status of HLCs, focusing on stem cell sources, the differentiation protocol for HLCs, the general characterization of HLCs, the generation of more functional HLCs, comparison with primary hepatocytes, and HLCs in cell-transplantation-based liver regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organ liver transplantations are performed worldwide for severe liver disease; however, they are associated with problems including donor shortage, surgical invasiveness, immune rejection, and high costs. On the other hand, hepatocyte transplantation has been performed as a bridging therapy, mainly in Europe and the United States, for patients with metabolic disorder syndrome or acute liver failure. Primary human hepatocytes offer immediate resources for studying liver diseases and transplantation. Although different culture systems that enable long-term culture and expansion of both rodent and human primary hepatocytes have been identified [1,2,3], the capacity of expansion is still limited and has donor-dependent variability. Furthermore, hepatocyte transplantation is associated with problems, such as donor shortage, reduced viability from techniques of isolation or cryo-preservation, lower long-term cell survival after transplantation, and increased portal pressure predisposing to portal embolism. Therefore, the fundamental solution lies in the development of new donor sources.
The current donor sources of hepatocytes include induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), and mesenchymal stem cells (MSCs). Hepatocyte-like cells (HLCs) have been differentiated worldwide from those stem cell sources as alternative primary hepatocytes. Despite the efforts of many investigators, the techniques available are still not able to differentiate HLCs to the level of normal adult primary hepatocytes. The functions of hepatocytes include gluconeogenesis, glycogen storage, glucose synthesis, lipid metabolism, blood protein synthesis and secretion, cholesterol metabolism, bile acid production, bile excretion, detoxification, ammonia metabolism, alcohol metabolism, ketone synthesis, and body temperature regulation. Differentiated HLCs do not attain all those functions or reach the same levels of primary hepatocytes. This review summarizes the current status of HLCs, comparing them with adult primary hepatocytes.
Stem cell sources
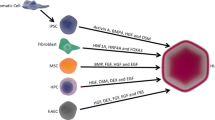
Stem cells have the features to generate various functional cells that can replicate themselves. Three stem cell sources of HLCs have been identified (Table 1). ESCs have potential pluripotency comparable to that of fertilized eggs and can produce all functional cells [4, 5]. It is possible for ESCs to self-renew while maintaining pluripotency and they are a source for producing HLCs in large quantities; however, the fact that they are produced from fertilized human eggs poses ethical issues. Yamanaka et al. [6, 7] established iPSCs in 2006 and 2007. The discovery that coordinated expression of a limited number of genes can re-program differentiated somatic cells to iPSCs has opened novel possibilities for developing cell-based models of diseases and re-generative medicine utilizing cell re-programming or cell transplantation. An ideal and distinctive potential of iPSC technology exists in their use in made-to-order therapies with autologous cells. Despite the potential benefits of autologous iPSC therapies, they have some limitations. First, the preparation of autologous iPSCs from each patient is costly. Second, because it takes more than 3 months to generate iPSCs, it is not possible to achieve timely effective treatment of some disorders from the aspect of early clinical use. Therefore, the most realistic method for iPSCs therapy is based on the collection of iPSC stock from various HLA-homozygous donors. Some HLA-homozygous iPSC bank projects have been prepared and maintained in Japan, Europe, and USA, including the European Bank for induced pluripotent Stem Cells (EBiSC); Kyoto University Stem Cell Bank, Japan; StemBANCC, EU; HipSci, UK; and the Coriell Institute, USA. Such iPSCs banks could potentially provide HLA-compatible normal iPSCs for individual recipients within a short time and at a much lower cost. MSCs represent another candidate for stem cell therapy and can be obtained from many different sources in the human body, such as bone marrow, umbilical cord, blood, amniotic fluid, scalp tissue, placenta, and adipose tissue [8, 9]. MSCs possess both multi-potentiality and semi-infinite proliferation ability. Regenerative medicine has already been performed using MSCs because they can be obtained relatively easily from autologous tissues, but further studies on their safety and efficacy are needed to promote their practical use.
The major question is, “Which cell sources can achieve differentiation of the most functional HLCs?” Jeong J et al. [10] reported that ESCs and iPSCs had similar abilities to differentiate into HLCs both in vitro (gene expression, the hepatocyte functions of accumulation of glycogen, secretion of albumin, and uptake of indocyanine green) and in vivo (transplantation into a damaged liver). A comparison of human iPSC-derived cardiomyocytes with human MSCs in an animal model of acute myocardial infarction has already been reported, and there were no functional differences between the iPSC and the MSC in the in vivo study [11]. Similar results were obtained in a corneal injury model animal using both iPSCs and MSCs [12]. On the other hand, the potential of iPSCs to differentiate into insulin-producing cells was reported to be significantly higher than that of human MSCs [13]. Therefore, no clear advantage has been proven among ESCs, iPSCs, and MSCs for the differentiation of HLCs [14,15,16]. MSC-based therapies are probably suitable for clinical application without the need for gene manipulation and will avoid ethical issues [17].
Differentiation protocol of HLCs
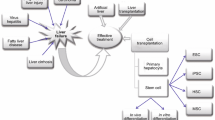
Hepatocyte differentiation technology has improved remarkably in the last decade. Most hepatocyte differentiation protocols can be divided into three differentiation steps: definitive endoderm differentiation, hepatoblast differentiation, and hepatocyte differentiation. Each of these differentiation steps is performed using the growth factors and cytokines known to be necessary for liver development. However, simpler and more cost-effective protocols have also reported without any growth factor or cytokines, using glycogen synthase kinase 3 (GSK3) inhibitors and dimethyl sulfoxide (DMSO) [18] (Fig. 1).
Differentiation protocol for hepatocyte-like cells (HLCs). Most HLC differentiation protocols can be divided into three differentiation steps: definitive endoderm differentiation, hepatoblast differentiation, and hepatocyte differentiation using the growth factors and cytokines known to be necessary for liver development.
Definitive endoderm differentiation
D’Amour et al. reported that high-purity definitive endoderm cells could be differentiated from human ESCs in the presence of activin A and low serum [19]. The combination of activin A and Wnt3a is often used to generate definitive endoderm cells with high hepatocyte differentiation capacity [20, 21]. To generate definitive endoderm cells with much higher hepatocyte differentiation capacity, Chen et al. used hepatocyte growth factor (HGF) in the presence of activin A and Wnt3a [22]. In addition to Wnt3a, the phosphoinositide 3-kinase (PI3K) inhibitor is sometimes used in definitive endoderm differentiation, but both positive and negative effects on definitive endoderm differentiation have been reported [23, 24]. Furthermore, instead of activin A and Wnt3a, activation of Wnt/β-catenin signaling via GSK3 inhibitors alone may achieve more functional HLCs from MSCs [25].
Hepatoblast differentiation
Fibroblast growth factors (FGF) and bone morphogenetic proteins (BMP) play important roles in liver specification. Cai et al. reported that the combination of FGF4 and BMP2-induced efficient hepatoblast differentiation from definitive endoderm cells [26]. Brolén et al. used the combination of BMP2/4 and FGF1/2/4 for hepatoblast differentiation [27]. Interestingly, a low concentration of FGF2 was found to promote hepatocyte differentiation, whereas an intermediate or high concentration of FGF2 promoted pancreatic or intestinal differentiation, respectively [28]. Therefore, the concentration of FGF should be strictly controlled. DMSO, which modifies histone acetylation, is also sometimes used in hepatoblast differentiation [29, 30].
Hepatocyte differentiation
Hepatoblasts can differentiate into hepatocytes and cholangiocytes. As HGF and oncostatin M (OsM) play important roles in liver development, most of the available protocols use HGF and OsM during the hepatocyte differentiation process [31, 32]. Dexamethasone (Dex) is also an important mediator during hepatic maturation [33].
General characterization of HLCs
As stem cells differentiate into definitive endoderm, hepatoblast-like cells, and HLCs, differentiation stage-specific markers are expressed (Fig. 2) [34]. Completion of HLCs is usually confirmed by gene expressions and function tests of the matured hepatocytes. Hepatocytes have many functions, and Table 2 shows the several parameters previously reported, namely synthetic function, lipid metabolism, glucose metabolism, urea metabolism, bile production and metabolism, cytochrome P450 function, and drug metabolism assays [35,36,37,38,39,40].
Generation of more functional HLCs
None of the many hepatic functions of HLCs are as strong as those in primary human hepatocytes. HLCs also express high levels of immature hepatocyte markers, such as alpha fetoprotein (AFP), suggesting a persistent immature/fetal phenotype [41, 42]. Therefore, many researchers have searched for ways that could mature HLCs.
Addition of some compounds
Ogawa et al. found that stimulation of cAMP signaling promoted the maturation of hepatoblast-like cells to HLCs [43]. Kotaka et al. also reported the addition of adrenergic receptor agonists in the maturation of hepatoblast-like cells to HLCs [44]. Kondo et al. reported that expression levels of drug metabolic enzymes were increased by valproic acid treatment [45]. Insulin-like growth factor-I (IGF-1) was found to promote hepatic differentiation [46]. Shan et al. identified the small molecular compounds, FPH1 and FH1, which can increase and decrease the percentage of CYP3A4- and AFP-positive cells, respectively [47]. This suggests that FPH1 and FH1 promote the hepatocyte differentiation toward a mature phenotype (Fig. 3).
Gene manipulation
Takayama et al. [48,49,50] demonstrated that definitive endoderm, hepatoblast, and hepatocyte differentiations were promoted by AdK7-mediated overexpression of SRY-related HMG-box 17 (SOX17), hematopoietically expressed homeobox (HEX), and hepatocyte nuclear factor (HNF) 4a, respectively. To further promote maturation of human HLCs, the combination of two transcription factors, forkhead box A2 (FOXA2) and HNF1a, made hepatic differentiation from human ESCs/iPSCs more efficient than the previous protocol [51]. The gene expression levels of the drug metabolic enzymes of AdK7-FOXA2- and HNF1a-transduced human ES/iPS-HLCs were similar to those of primary human hepatocytes. Moreover, approximately 90% of AdK7-FOXA2- and HNF1a-transduced human ES/iPS-HLCs were albumin (ALB)-positive cells. Sasaki et al. reported that HNF6 overexpression enhanced CYP3A4 expression levels in human iPS-HLCs [52]. These findings suggest that the overexpression of hepatic transcription factors is powerful for generating metabolically functional HLCs (Fig. 3).
3D culture/organoids/co-culture
Recently, 3D cell culture and co-culture systems were adopted to maintain and enhance the liver-specific functions of HLCs. The hepatic functionality of HLCs was enhanced using 3D cell culture devices, such as a hollow fiber-based 3D perfusion bioreactor [53], a Nanopillar plate [54], a micro carrier culture system [55], micro stencil.
array [56], and a RAFT 3D Cell Culture System [57]. Furthermore, hepatocyte-like liver organoids induced by iPSCs with 3D culture had more functionality than the usual 3D HLCs [58]. Pettinato G et al. reported that hepatic organoids from iPSCs with endothelial cells became more functional [59].
The hepatic functionality of HLCs could also be enhanced by co-culturing
with Swiss 3T3 cells [60], human iPS cell-derived endothelial cells [61], 3T3-J2 murine embryonic fibroblasts [62], human umbilical vein endothelial cells, and adipose-derived stem cells [63]. To enhance the hepatic functionality of HLCs further, a 3D co-culture system is needed to better mimic the hepatocyte environment in vivo. It was also reported that hepatectomized patient serum could promote hepatocyte differentiation from human iPSCs [64]. This fact suggests that hepatic maturation might be promoted by soluble factors that exist under conditions, such as liver injury.
Current status of HLCs: comparison with primary hepatocytes
As described above, hepatocyte differentiation technology has been improved with the addition of compounds, gene manipulation, and 3D culture. Table 3 summarizes the power of the currently modified HLCs compared with that of primary hepatocytes. Normal HLCs have 40% less hepatic function (including synthetic function, urea production, and CYP activity) than primary hepatocytes [34, 36, 41]. When certain compounds are added to the differentiation cocktail, it increases to 60–80% for albumin synthesis and 40–100% for CYP activity [43,44,45,46,47]. Gene manipulation is advantaged by CYP activity, with both CYP3A4 and 1A2 activity reaching the levels of primary hepatocytes [48,49,50,51,52]. Spheroids or organoids with a 3D culture system also improve hepatic functions and AAT synthesis or urea production are reported to be superior to those of primary hepatocytes [53,54,55,56,57,58,59]. When those spheroids were transplanted in vivo, angiogenesis around the transplanted cells was stimulated by increasing VEGF expression [65]. Furthermore, 3D spheroids were reported to improve engraftment of transplanted cells, and the main factors involved in the mechanism that improved engraftment of the transplanted cells were the inhibition of cell anoikis through a scaffold and the avoidance of central necrosis within cell aggregates [66]. The 3D shape also enabled transplanted cells to release various cytokines, making gaps or spaces between cells [66, 67]. In terms of the clinical application of regenerative medicine, 3D HLCs seem to have an advantage in transplantation. HLCs with gene manipulation can be used for pharmaceutical research because of those high CYP activities. In the future, HLCs with high urea metabolism will be necessary for patients with metabolic disorder syndrome. On the other hand, the differentiation of HLCs in vitro alone does not result in complete hepatic maturation. When some induced differentiated cells start to mature, those cells express some receptors in relation to the surrounding cells after being transplanted in vivo [68]. A niche is formed with not only parenchymal cells but also kupffer cells, stellate cells, endothelial cells, and immune cells in the liver, and those cells are closely related to each other [69]. Therefore, some intervention after HLC transplantation is thought to be required for final maturation in vivo.
HLCs in cell-transplantation-based liver regeneration
A major hurdle for the application of HLCs in regenerative medicine is the inefficient engraftment and the low level of subsequent proliferation required for substantial re-population of the liver. Primary hepatocytes in the host liver are highly capable of proliferating in response to proliferative stimuli. Because hepatocytes in most inherited liver-based metabolic disorders retain normal proliferative capacity, transplanted HLCs need not only to penetrate through the sinusoidal endothelial barrier to engraft, but they also need to compete with the host hepatocytes to re-populate the liver. Therefore, some preparative manipulations of host cells are required to provide a proliferative advantage to the transplanted cells. One preparative regimen developed for use in rat and mouse transplant recipients consists of administering retrorsine, a plant alkaloid that inhibits hepatocyte replication and 70% hepatectomy to stimulate cell division [68]. Another preparative regimen with potential for clinical translation consists of X-irradiation of a portion of the recipient liver, followed by the transplantation of HLCs [69].
It is also important to establish whether transplanted cells can proliferate gradually. It is widely recognized that transplanted cells should be replaced in vivo to achieve the maturation process gradually, and not by rapidly creating a space for proliferation, such as by hepatectomy or X-ray irradiation [70]. To improve transplantation efficiency, several ectopic sites have been investigated, including spleen, peritoneal cavity, kidney, lung, pancreas, and fat pads [68, 71,72,73,74,75,76] (Table 4). Bioengineering approaches have also been applied in cell transplantation. Song et al. [75] transplanted HLCs in immuno-competent mice via 3D cell co-aggregates with stromal cells and encapsulation. This study demonstrated an improved approach for the engraftment of HLCs with both ectopic transplantation sites and bioengineering approaches. Nagamoto et al. [76] used cell-sheet engineering technology by attaching HLC sheets onto the surface of the liver of a mouse with acute liver failure, resulting in improved hepatocyte engraftment and animal survival.
Another major hurdle for the application of HLCs involves establishing whether iPSCs or MSCs, isolated from patients with inherited disorders, could be differentiated into normal HLCs. HLCs have been generated from patients with various inherited disorders [77,78,79,80,81,82,83,84], such as AAT deficiency (ATD), familial hypercholesterolemia (abnormalities of low-density lipoprotein receptor (LDLR)), autosomal-dominant hypercholesterolemia (gain-of-function mutation of proprotein convertase subtilisin kexin type 9 (PCSK9)), Wilson’s disease (ATP7B deficiency), familial transthyretin amyloidosis (FTA), glycogen storage disease type 1a (GSD1A, glucose-6-phosphatase deficiency), Crigler–Najjar syndrome type 1 (CN1, UGT1A1 deficiency), and primary hyperoxaluria-1 (PH1, AGXT deficiency). The therapeutic effect of HLCs in all patients with inherited disorders was not strong enough to ameliorate the effects of these diseases. With that in mind, the banks of lines of iPSCs with an extensive range of HLA profiles are being developed with the participation of many nations including the European Bank for induced pluripotent Stem Cells (EBiSC); the Kyoto University Stem Cell Bank, Japan; StemBANCC, EU; HipSci, UK; and the Coriell Institute, USA. An iPSC bank could potentially provide HLA-compatible normal iPSCs for individual recipients in a short time and at a much lower cost.
The results of this study highlight the following:
-
(1)
A clear advantage has not been proven among ESCs, iPSCs, and MSCs for the differentiation of HLCs.
-
(2)
Hepatocyte differentiation protocols can be divided into three steps: definitive endoderm differentiation, hepatoblast differentiation, and hepatocyte differentiation with key cytokines and growth factors.
-
(3)
The addition of some compounds, gene manipulation, and 3D culture (or co-culture) induce more functional HLCs.
-
(4)
3D HLCs seem to be best in terms of the clinical application of regenerative medicine. HLCs with gene manipulation can be used for pharmaceutical research because of their high CYP activity.
-
(5)
A major hurdle for the application of HLCs transplantation is the inefficient engraftment and low level of subsequent proliferation. Transplantation is not recommended for HLC patients with inherited disorders without effective therapeutic improvement of their disease.
HLCs for clinical application
Although there have been some clinical trials on using undifferentiated MSCs for both acute and chronic liver disease [85,86,87], the clinical application of differentiated HLCs is still in progress. A case report of the allogeneic transplantation of bone marrow MSC-derived HLCs in a patient with familial hypercholesterolemia (FH) was published in 2010 [88]. The recipient was a 12-year-old girl with FH, for whom a total of 4.0 × 108 cells, including HLCs (3.0 × 108 cells) and undifferentiated MSCs (0.5 × 108 cells), were infused through the portal vein catheter under ultrasound guidance. Transplantation of the HLCs was safe and feasible; however, it did not correct the metabolic abnormality. The HLCs did not repopulate within the liver and the authors speculated that the number of transplanted cells should be increased to around 5 billion and that strategies to improve the long-term implantation of HLCs into the host liver should be addressed.
There was also a report of a phase I/II clinical study on the autologous transplantation of bone marrow MSC-derived HLCs in patients with liver cirrhosis (HCV) [89]. In this study, 2.0 × 108 immature HLCs (only 7 days alone for differentiation) were injected for intrasplenic or intrahepatic transplantation. The HLC transplantation showed short-term efficacy reducing the amount of ascites and improving lower limb edema and serum albumin levels. The authors concluded that the long-term efficacy of HLCs should be investigated further.
In 2020, a successful case of the transplantation of HLSs from ESCs in a patient with inborn urea cycle disorder was described by the National Center for Child Health and Development, Tokyo, Japan (not published yet). In that case, HLC transplantation was performed as bridging therapy following liver transplantation. This might be the world's first report of using HLSs from ESCs. Further clinical study is necessary to standardize the cell dose, determine the life span of the injected cells, and identify signs of long-term efficacy.
In conclusion, the directed differentiation of stem cells toward the mature hepatocyte phenotype requires further refinement. There has been some success in re-populating the livers of rodent models of human diseases with HLCs; however, the initial engraftment and subsequent proliferation of the transplanted HLCs remain low. Research by many groups worldwide continues to yield creative and promising solutions to eventually overcoming the existing hurdles to applying HLCs in personalized regenerative medicine.
Abbreviations
- HLCs:
-
Hepatocyte-like cells
- IPSCs:
-
Induced pluripotent stem cells
- ESCs:
-
Embryonic stem cells
- MSCs:
-
Mesenchymal stem cells
- HGF:
-
Hepatocyte growth factor
- PI3K:
-
Phosphoinositide 3-kinase
- GSK3:
-
Glycogen synthase kinase 3
- FGF:
-
Fibroblast growth factors
- BMP:
-
Bone morphogenetic proteins
- DMSO:
-
Dimethyl sulfoxide
- OsM:
-
Oncostatin M
- Dex:
-
Dexamethasone
- AFP:
-
Alpha fetoprotein
- IGF-1:
-
Insulin-like growth factor-I
- SOX17:
-
SRY-Box 17
- HEX:
-
Hematopoietically expressed homeobox
- HNF:
-
Hepatocyte nuclear factor
- FOXA2:
-
Forkhead box A2
- ALB:
-
Albumin
- ATD:
-
AAT deficiency
- LDLR:
-
Low-density lipoprotein receptor
- PCSK9:
-
Proprotein convertase subtilisin kexin type 9
- FTA:
-
Familial transthyretin amyloidosis
- GSD1A:
-
Glycogen storage disease type 1a
- CN1:
-
Crigler-Najjar syndrome type 1
- PH1:
-
Primary hyperoxaluria-1
- EBiSC:
-
European Bank for induced pluripotent Stem Cells
- FH:
-
Familial hypercholesterolemia
References
Zhang K, Zhang L, Liu W, Ma X, Cen J, Sun Z, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018;23:806–819.e4.
Fu GB, Huang WJ, Zeng M, Zhou X, Wu HP, Liu CC, et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29:8–22.
Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018;175:1607–1619.e15.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Saito Y, Shimada M, Utsunomiya T, Ikemoto T, Yamada S, Morine Y, et al. The protective effect of adipose-derived stem cells against liver injury by trophic molecules. J Surg Res. 2013;180(1):162–8.
Saito Y, Shimada M, Utsunomiya T, Ikemoto T, Yamada S, Morine Y, et al. Homing effect of adipose-derived stem cells to the injured liver: the shift of stromal cell-derived factor 1 expressions. J Hepatobiliary Pancreat Sci. 2014;21(12):873–80.
Jeong J, Kim KN, Chung MS, Kim HJ. Functional comparison of human embryonic stem cells and induced pluripotent stem cells as sources of hepatocyte-like cells. Tissue Eng Regen Med. 2016;13(6):740–9.
Citro L, Naidu S, Hassan F, Kuppusamy ML, Kuppusamy P, Angelos MG, et al. Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction. PLoS ONE. 2014;9(12):e116281.
Yun YI, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, et al. Comparison of the anti-inflammatory effects of induced pluripotent stem cell-derived and bone marrow-derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy. 2017;19(1):28–35.
Abazari MF, Nasiri N, Nejati F, Zare Karizi S, Amini Faskhodi M, Saburi E, et al. Comparison of human-induced pluripotent stem cells and mesenchymal stem cell differentiation potential to insulin producing cells in 2D and 3D culture systems in vitro. J Cell Physiol. 2020;235(5):4239–46.
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4.
Tolosa L, Caron J, Hannoun Z, Antoni M, López S, Burks D, et al. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res Ther. 2015;6:246.
Yin L, Zhu Y, Yang J, Ni Y, Zhou Z, Chen Y, et al. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol Med Rep. 2015;11:1722–32.
Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T. Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther. 2010 Jun;5(2):182–9.
Cong Du, Feng Y, Qiu D, Yan Xu, Pang M, Cai N, et al. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res Ther. 2018;9:58.
D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–41.
Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105:12301–6.
Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, et al. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res. 2013;319:2535–44.
Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193–203.
Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–65.
Magner NL, Jung Y, Wu J, Nolta JA, Zern MA, Zhou P. Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cells. 2013;31:2095–103.
Huang J, Guo X, Li W, Zhang H. Activation of Wnt/β-catenin signalling via GSK3 inhibitors direct differentiation of human adipose stem cells into functional hepatocytes. Sci Rep. 2017;17(7):40716.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Brolen G, Sivertsson L, Bjorquist P, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–94.
Brolén G, Sivertsson L, Björquist P, Eriksson G, Ek M, Semb H, et al. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56.
Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902.
Kajiwara M, Aoi T, Okita K, Takahashi R, Inoue H, Takayama N, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12538–43.
Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–89.
Snykers S, De Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605.
Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26(5):1117–27.
Takayama K, Hagihara Y, Toba Y, Sekiguchi K, Sakurai F, Mizuguchi H. Enrichment of high-functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research. Biomaterials. 2018;161:24–322.
Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN, et al. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci USA. 2011;108(29):11842–7.
Si-Tayeb K. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305.
Cayo MA, Cai J, DeLaForest A, Noto FK, Nagaoka M, Clark BS, et al. ‘JD’ iPS cell-derived hepatocytes faithfully recapitulate the pathophysiology of familial hypercholesterolemia. Hepatology. 2012;56(6):2163–71.
Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26(1):120–6.
Tada K, Roy-Chowdhury N, Prasad V, Kim BH, Manchikalapudi P, Fox IJ, et al. Long-term amelioration of bilirubin glucuronidation defect in Gunn rats by transplanting genetically modified immortalized autologous hepatocytes. Cell Transplant. 1998;7(6):607–16.
Murray JW, Thosani AJ, Wang P, Wolkoff AW. Heterogeneous accumulation of fluorescent bile acids in primary rat hepatocytes does not correlate with their homogenous expression of ntcp. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G60–G6868.
Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, et al. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Res. 2012;9:196e207.
Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581e9.
Ogawa S, Surapisitchat J, Virtanen C, Ogawa M, Niapour M, Sugamori KS, et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 3285e;140:3285e96.
Kotaka M, Toyoda T, Yasuda K, Kitano Y, Okada C, Ohta A, et al. Adrenergic receptor agonists induce the differentiation of pluripotent stem cell-derived hepatoblasts into hepatocyte-like cells. Sci Rep. 2017;7(1):16734.
Kondo Y, Iwao T, Yoshihashi S, Mimori K, Ogihara R, Nagata K, et al. Histone deacetylase inhibitor valproic acid promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells. PLoS ONE. 2014;9:e104010.
Shabani Azandaryani Z, Davoodian N, Samiei A, Rouzbehan S. Insulin-like growth factor-I promotes hepatic differentiation of human adipose tissue-derived stem cells. Cell Biol Int. 2019;43(5):476–85.
Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA, et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514e20.
Takayama K, Inamura M, Kawabata K, Tashiro K, Katayama K, Sakurai F, et al. Efficient and directive generation of two distinct endoderm lineages from human ESCs and iPSCs by differentiation stage-specific SOX17 transduction. PLoS ONE. 2011;6:e21780.
Inamura M, Kawabata K, Takayama K, Tashiro K, Sakurai F, Katayama K, et al. Efficient generation of hepatoblasts from human ES cells and iPS cells by transient overexpression of homeobox gene HEX. Mol Ther. 2011;19:400e7.
Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4alpha transduction. Mol Ther. 2012;20:127e37.
Takayama K, Inamura M, Kawabata K, Sugawara M, Kikuchi K, Higuchi M, et al. Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1alpha transduction. J Hepatol. 2012;57:628e36.
Sasaki T, Takahashi S, Numata Y, Narita M, Tanaka Y, Kumagai T, et al. Hepatocyte nuclear factor 6 activates the transcription of CYP3A4 in hepatocytelike cells differentiated from human induced pluripotent stem cells. Drug Metab Pharmacokinet. 2013;28:250e9.
Miki T, Ring A, Gerlach J. Hepatic differentiation of human embryonic stem cells is promoted by three-dimensional dynamic perfusion culture conditions. Tissue Eng Part C Methods. 2011;17:557e68.
Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials. 1781e;34:1781e9.
Park Y, Chen Y, Ordovas L, Verfaillie CM. Hepatic differentiation of human embryonic stem cells on microcarriers. J Biotechnol. 2014;174:39e48.
Yao R, Wang J, Li X, Jung Jung D, Qi H, Kee KK, et al. Hepatic differentiation of human embryonic stem cells as microscaled multilayered colonies leading to enhanced homogeneity and maturation. Small. 4311e;10:4311e23.
Gieseck RL 3rd, Hannan NR, Bort R, Hanley NA, Drake RA, Cameron GW, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS ONE. 2014;9:e86372.
Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5):970–85.
Pettinato G, Lehoux S, Ramanathan R, Salem MM, He LX, Muse O, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with endothelial Cells. Sci Rep. 2019;9(1):8920.
Nagamoto Y, Tashiro K, Takayama K, Ohashi K, Kawabata K, Sakurai F, et al. The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3T3 cell sheets. Biomaterials. 4526e;33:4526e34.
Du C, Narayanan K, Leong MF, Wan AC. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 6006e;35:6006e14.
Berger DR, Ware BR, Davidson MD, et al. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology. 1370e;61:1370e81.
Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA. 2206e;113:2206e11.
Xing Q, Luo Y, Gao Y, Zhang S, Zhu Z, Wang Y, et al. Hepatectomised patient sera promote hepatocyte differentiation of human-induced pluripotent stem cells. Dig Liver Dis. 2014;46:731e7.
Ikemoto T, Feng R, Iwahashi SI, Yamada S, Saito Y, Morine Y, et al. In vitro and in vivo effects of insulin-producing cells generated by xeno-antigen free 3D culture with RCP piece. Sci Rep. 2019;9:10759.
Nakamura K, Cell SA. Cell aggregate-like technology using recombinant peptide pieces for MSC transplantation. Curr Stem Cell Res Ther. 2019;14:52–6.
Iwazawa R, Kozakai S, Kitahashi T, Nakamura K, Hata K. The therapeutic effects of adipose-derived stem cells and recombinant peptide pieces on mouse model of DSS colitis. Cell Transplant. 2018;27(9):1390–400.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556(7700):239–43.
Saito Y, Morine Y, Shimada M. Mechanism of Impairment on Liver Regeneration in Elderly Patients: Role of Hepatic Stellate Cell Function. Hepatol Res. 2017;47(6):505–13.
Yoshizato K, Tateno C. In vivo modeling of human liver for pharmacological study using humanized mouse. Expert Opin Drug Metab Toxicol. 2009;5(11):1435–46.
Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, et al. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953–64.
Nagamoto Y, Takayama K, Tashiro K, Tateno C, Sakurai F, Tachibana M, et al. Efficient engraftment of human induced pluripotent stem cell-derived hepatocyte-like cells in uPA/SCID mice by overexpression of FNK, a Bcl-xL mutant gene. Cell Transplant. 2015;24:1127–38.
Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39.
Asgari S, Moslem M, Bagheri-Lankarani K, Pournasr B, Miryounesi M, Baharvand H. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev. 2013;9:493–504.
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–42.
Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068–75.
Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Investig. 2010;120(9):3127–36.
Tafaleng EN, Chakraborty S, Han B, Hale P, Wu W, Soto-Gutierrez A, et al. Induced pluripotent stem cells model personalized variations in liver disease resulting from alpha1-antitrypsin deficiency. Hepatology. 2015;62(1):147–57.
Choi SM, Kim Y, Shim JS, Park JT, Wang RH, Leach SD, et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57(6):2458–68.
Zhang S, Chen S, Li W, Guo X, Zhao P, Xu J, et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson’s disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet. 2011;20(16):3176–87.
Brown MS, Goldstein JL. Biomedicine lowering LDL—not only how low, but how long? Science. 2006;311(5768):1721–3.
Cayo MA, Cai J, DeLaForest A, Noto FK, Nagaoka M, Clark BS, et al. JD induced pluripotent stem cell-derived hepatocytes faithfully recapitulate the pathophysiology of familial hypercholesterolemia. Hepatology. 2012;56(6):2163–71.
Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–36.
Leung A, Nah SK, Reid W, Ebata A, Koch CM, Monti S, et al. Induced pluripotent stem cell modeling of multisystemic, hereditary transthyretin amyloidosis. Stem Cell Rep. 2013;1(5):451–63.
Smets F, Dobbelaere D, McKiernan P, Dionisi-Vici C, Broué P, Jacquemin E, et al. Phase I/II trial of liver-derived mesenchymal stem cells in pediatric liver-based metabolic disorders: a prospective, open label, multicenter, partially randomized, safety study of one cycle of heterologous human adult liver-derived progenitor cells (hepastem) in urea cycle disorders and crigler-najjar syndrome patients. Transplantation. 2019 Sep;103(9):1903–15.
Wen-Xiong Xu, He H-L, Pan S-W, Chen Y-L, Zhang M-L, Zhu S, et al. Combination treatments of plasma exchange and umbilical cord-derived mesenchymal stem cell transplantation for patients with hepatitis B virus-related acute-on-chronic liver failure: a clinical trial in China. Stem Cells Int. 2019;4(2019):4130757.
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66(1):209–19.
Mohamadnejad M, Pournasr B, Bagheri M, Aghdami N, Shahsavani M, Hosseini LA, et al. Transplantation of allogeneic bone marrow mesenchymal stromal cell-derived hepatocyte-like cells in homozygous familial hypercholesterolemia. Cytotherapy. 2010;12(4):566–8.
Amer M-E, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;10:936–41.
Funding
No funding
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
Yu Saito and the other co-authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saito, Y., Ikemoto, T., Morine, Y. et al. Current status of hepatocyte-like cell therapy from stem cells. Surg Today 51, 340–349 (2021). https://doi.org/10.1007/s00595-020-02092-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02092-6