Abstract
Purpose
The coexistence of sarcopenia is associated with postoperative complications, including infection after abdominal surgery. We evaluated the association between sarcopenia and surgical site infection (SSI) after surgery for ulcerative colitis.
Methods
The subjects of this retrospective study were 69 patients who underwent restorative proctocolectomy with perioperative abdominal computed tomography (CT). Sarcopenia was diagnosed by measuring the cross-sectional area of the right and left psoas muscles as the total psoas muscle area on CT images. We assessed whether sarcopenia was associated with SSI and clinical factors, including nutritional and inflammatory markers.
Results
The lowest quartiles defined as sarcopenia in men and women were 567.4 and 355.8 mm2/m2, respectively. According to this classification, 12 men and 6 women had sarcopenia. Patients with sarcopenia had a lower body mass index (p = 0.0004) and a higher C-reactive protein concentration (p = 0.05) than those without sarcopenia. SSIs were identified in 12 patients (17.3 %) and included six pelvic abscesses and seven wound infections. According to multivariate analysis, sarcopenia was an independent risk factor for SSI (odds ratio = 4.91, 95 % confidence interval 1.09–23.5, p = 0.03).
Conclusion
Sarcopenia is predictive of SSI after pouch surgery for ulcerative colitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Restorative proctocolectomy with ileal pouch–anal anastomosis (IPAA) is typically performed for patients with ulcerative colitis (UC) [1]. However, patients with UC who undergo IPAA are at risk of the development of several septic complications [2, 3], especially surgical site infection (SSI), a nosocomial complication surgical patients are susceptible to [4–8]. Even incisional SSI can prolong the hospital stay and increase medical expenses [9]. Pelvic sepsis, an intra-abdominal septic complication caused by pelvic abscess formation, including organ/space SSI, is most likely to be associated with breakdown of the IPAA, which may result in pouch failure caused by a persistent fistula and anal dysfunction [10]. Thus, an understanding of the factors predictive of SSI may assist in formulating a surgical plan and could be useful markers for avoiding compromised quality of life after pouch surgery.
In 1991, the National Nosocomial Infections Surveillance System of the Centers for Disease Control and Prevention (CDC) proposed a risk index based on calculating a score for each operation and counting the number of risk factors for SSI [11]. Although this risk index is widely used and comprehensive, it is not disease-specific. Postoperative complications, including SSI, after colorectal surgery may be associated with preoperative factors, such as age, sex, malnutrition, prior surgery, comorbidities, obesity, and malignant disease [12].
Sarcopenia is a condition characterized by loss of skeletal muscle mass and muscle strength [13]. In 1995, Rosenberg and Roubenoff [14] described sarcopenia as an age-related decrease in skeletal muscle mass and function associated with advanced organ failure of the heart, lung, liver, and kidney, with malnutrition, inflammatory disease, endocrine disease, and malignancy [13, 15]. Based on the findings of cross-sectional imaging or bioelectrical impedance analysis, the clinical impacts of sarcopenia on patients with nonmalignant conditions or malignant diseases include functional impairment, disability [16], acceleration of chemotherapeutic toxicity [17–19], and poor survival [20–25]. Postoperative infection has also been associated with sarcopenia in abdominal surgery, including colorectal surgery [23, 26] and liver transplantation [27]. However, to our knowledge, no study has evaluated the relationship between sarcopenia and postoperative complications in patients with UC. We hypothesized that sarcopenia is a risk factor for SSI in patients undergoing pouch surgery for UC because of their malnutrition caused by this debilitating illness, with chronic inflammation and inadequate nutrient intake. The aim of this study was to evaluate the association between sarcopenia and SSI in patients with UC.
Methods
Patients and clinical data collection
This study was performed in accordance with the Declaration of Helsinki and was approved by our institutional review board. All patients gave written informed consent to participate in the study. A total of 250 patients underwent two- or three-stage ileal J-pouch–anal anastomosis (IPAA) at Mie University Hospital between 2000 and 2013. The diagnosis and assessment of UC were based on the patients’ clinical, radiographic, endoscopic, and pathological data. Patients aged ≤14 years (n = 32), those with colitis-associated cancer (n = 18), and those who underwent emergency surgery (n = 2) were excluded from this study to reduce clinical bias. Finally, 69 of 198 patients who had undergone perioperative abdominal computed tomography (CT) scans (from 30 days prior to surgery to within 7 days of surgery) were included in this study. The following clinical characteristics were recorded: sex, age at operation, preoperative duration from diagnosis, total prednisolone dose within 1 month before surgery, preoperative body mass index (BMI), preoperative laboratory data [total protein (TP) concentration, cholinesterase (Ch-E) concentration (ΔpH), albumin concentration, lymphocyte count, and C-reactive protein (CRP) concentration], operation time, operative blood loss, prior colectomy, and postoperative infection. The prognostic nutritional index (PNI) was also evaluated as a nutritional factor [28]. The PNI was calculated based on the admission data as follows: 10 × serum albumin concentration (g/dl) + 0.005 × total lymphocyte count (per mm3). We evaluated the incidence of SSI developing within 30 days of surgery. SSI includes superficial incisional, deep incisional (soft tissue), or organ/space infection, and was classified according to the criteria of the CDC [11]. Organ/space SSI included anastomotic leakage or intra-abdominal abscess formation, which was defined as septic fluid in the peritoneal space, proven by surgical drainage or needle aspiration and bacteriologic culture. Incisional SSI included wound infections demonstrated by purulent fluid or pus in the wound incision. Hyperemia and local warming at the surgical site was also accepted as a criterion for superficial incisional SSI. Postoperative anastomotic leakage was diagnosed by performing a colonic enema.
All patients received a 3-day course of a second-generation cephalosporin antibiotic (cefmetazole) for prophylaxis. Infusions were started soon after the induction of anesthesia to ensure that a peak blood level of the antibiotic was obtained during surgery. Further doses of the antibiotic were given every 3 h during the operation.
Image analysis
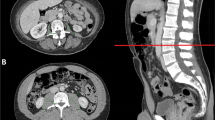
Body composition was evaluated by measuring the cross-sectional area of the right and left psoas muscles as the total psoas muscle area (TPA) on CT images [22, 24, 29]. The fourth lumbar vertebra (L4) was chosen as a standard landmark, and TPA was measured at the superior aspect of L4. Muscles were identified on the basis of their anatomic features, and measurements were taken by semiautomated calculation with manual outlining of the borders of both psoas muscles and setting the density threshold at −30 to 110 Hounsfield units (HU). The psoas muscle area was calculated with exclusion of the vasculature and areas of fatty infiltration based on HU. The measured psoas area was finally normalized for height as a convention for body composition measurements (TPA mm2/m2) [22, 24, 30]. Sarcopenia was defined as a value up to the lowest TPA quartile for men and women separately.
Statistical analysis
All data were entered into a database and analyzed using the JMP 7 software program (SAS Institute, Cary, NC, USA). Quantitative data are presented as mean ± standard deviation. Data were compared between patients with and those without sarcopenia, using the Wilcoxon test for each sex individually. Correlations between TPA and preoperative factors were analyzed by Spearman’s correlation. Univariate analysis was used to examine the relationship between sarcopenia and the variables studied. All variables associated with sarcopenia with a p value of <0.1 on univariate analysis were examined consecutively by multivariate logistic regression analysis. A p value of <0.05 was considered significant. Cutoff values in the logistic regression analysis were tested using a receiver operating characteristic curve for steroid dose, median operation time and blood loss, and the upper quartile for age. Cutoff values of serum TP, albumin, Ch-E, and CRP were defined according to the standard values. The PNI cutoff was taken from a previous report [28].
Results
Table 1 summarizes the clinical characteristics of the 69 patients who underwent preoperative CT scans and were the subjects of this study. Nineteen patients underwent subtotal colectomy before pouch surgery. The mean preoperative BMI was 20.4 kg/m2, and the mean monthly prednisolone dose in the 1 month prior to surgery was 274 mg. SSIs were identified in 12 patients (17.3 %) and included six organ/space SSIs, all of which were pelvic abscesses, and seven incisional SSIs. Five pelvic abscesses were complicated by anastomotic leakage. Figure 1 shows the distribution of the adjusted TPA, stratified by the sex of the patients: The mean TPA was higher in men (703 mm2/m2) than in women (456 mm2/m2, p < 0.001). The lowest sarcopenia quartiles for men and women separately were 567.4 and 355.8 mm2/m2, respectively. Twelve men and six women had sarcopenia. Table 2 examines the associations between sarcopenia and perioperative factors. The BMI was lower (p = 0.0004), and the CRP concentration was higher (p = 0.05) in patients with than in those without sarcopenia. The incidence of sarcopenia tended to be higher in patients who had three-stage IPAA than in those who had two-stage IPAA. Moreover, four patients (57.1 %) with incisional SSI and four (66.7 %) with organ/space SSI had sarcopenia, although the incidences of sarcopenia did not differ significantly between SSI grades. Risk factors for SSI in pouch surgery among all clinical factors, including sarcopenia, were evaluated by logistic regression analysis (Table 3). Age (≥50 years), steroid dose (≥300 mg), BMI (<18.5 kg/m2), CRP concentration (≥0.5 mg/dl), TP concentration (<6.5 g/dl), Ch-E concentration (<0.6 ΔpH), PNI (<45), prior colectomy, operative blood loss (≥300 g), operative time (≥340 min), and sarcopenia were candidate factors in univariate analysis. In univariate analysis, PNI [odds ratio (OR) = 4.87, 95 % confidence interval (95 % CI) = 1.34–20.4, p = 0.02] and sarcopenia (OR = 5.85, 95 % CI = 1.58–23.3, p = 0.008) were significant risk factors for postoperative infection. In multivariate analysis, sarcopenia was an independent risk factor for SSI (OR = 4.91, 95 % CI = 1.09–23.5, p = 0.03).
Discussion
Patients with UC have a high risk of SSI, especially deep incision and organ/space SSI [7, 8]. Several reports have identified several risk factors associated with SSI in patients with UC, including preoperative steroid use [31], perioperative transfusion [32], high American Society of Anesthesiologists physical status [8, 31], high BMI [33], hand-sewn anastomotic technique [34], and anemia [32]. To our knowledge, this study is the first to show an association between SSI and sarcopenia in patients with UC. Our findings demonstrated that sarcopenia is an independent risk factor for SSI in patients undergoing pouch surgery for UC. Sarcopenia is a good predictor of SSI, which is strongly associated with pelvic sepsis. Pelvic sepsis is, in turn, associated with poor quality of life following anal dysfunction. Therefore, sarcopenia may be an indicator of the optimal operative procedure, namely, total proctocolectomy with concurrent IPAA or subtotal colectomy followed by IPAA.
The reported risk factors for sarcopenia include age, physical performance, and nutritional and consumptive disease-related factors [13]. In fact, the patients with sarcopenia in the present study had a significantly lower BMI, attributed to their nutritional status, than patients without sarcopenia. In retrospective cohort studies, sarcopenia was associated with increased morbidity, longer hospital stay, and increased mortality in patients undergoing abdominal surgery for pancreatic cancer [22], colorectal cancer [23, 26], liver metastasis [24], liver transplantation [27], or hepatocellular carcinoma [35]. Lieffers et al. [23] reported that sarcopenia predicted the development of infection, requirement for inpatient rehabilitation care, and consequently, a longer hospital stay for patients undergoing colorectal surgery. They evaluated the correlation between sarcopenia and preoperative comorbidities, such as hypertension, anemia, and cardiac arrhythmias (excluding age and BMI), as well as postoperative outcomes of abdominal surgery [23]. In the present study, sarcopenia was not associated with age, although it was inversely correlated to age in previous reports [22, 23]. This difference may be explained by the younger cohort in our study, who were less likely to have muscle depletion associated with aging. UC patients also tend to be malnourished because of their low caloric intake, bleeding, and wasting associated with chronic inflammation, as well as sarcopenia. These factors are more strongly associated with nutritional status than age. Also, we evaluated the relationship between sarcopenia and operative procedures. Sarcopenic patients were more often treated with three-stage IPAA than two-stage IPAA, although patients with three-stage IPAA are generally in a good nutritional state after subtotal colectomy. This result suggests that patients who undergo three-stage IPAA do not have enough time to allow the recovery of skeletal muscle masses, as IPAA is performed a few months after subtotal colectomy, and sarcopenia was the only independent risk factor of SSI in our multivariate analysis including three-stage IPAA as a covariate.
In this study, we evaluated the correlation between sarcopenia and markers of malnutrition, such as hypoalbuminemia, a low serum Ch-E concentration, a low serum TP concentration, and a low BMI [36]. A few studies have evaluated the correlation between sarcopenia and nutritional or inflammatory markers, such as the serum albumin concentration [37] and CRP concentration [38, 39], in patients undergoing surgery. Sarcopenia was found to be associated with hypoalbuminemia, a low BMI, and a high serum CRP concentration in older persons [40]. Our study also showed that sarcopenia was associated with a high serum CRP concentration and low BMI, but nutritional markers, such as the serum albumin, Ch-E, and TP concentrations, were not associated with SSI in patients with UC undergoing pouch surgery. This result suggests that these nutritional markers, which are likely affected by alterations in blood constituents, such as those induced by dehydration, may undergo changes in a shorter period of time than skeletal muscle mass in patients with UC.
CT and magnetic resonance imaging are the gold standard diagnostic techniques for sarcopenia. Both are precise imaging systems, although several reports have described the use of absorptiometry and bioimpedance analysis for this purpose [13]. It is evident that the core muscle size, as determined using the psoas muscle or total skeletal muscle on an abdominal cross-sectional image, provides a good measure of the skeletal muscle mass, including increases or decreases [41]. The total abdominal muscle area (TAMA) or TPA, identified by applying skeletal muscle-specific HU in CT, can be measured at the level of L3 or L4 [41–43], because the skeletal muscle area 5 cm above the L4/L5 level has the highest correlation with the skeletal muscle volume in the total body. We measured the TPA using a semiautomated calculation with manual outlining of the borders of both psoas muscles, which is simple and readily available. In a systematic review, several cutoff values of the TPA are described, including the lowest tertile, the lowest quartile, and the cutoff value calculated by sensitivity analysis [41]. Furthermore, TPA and TAMA were normalized for patient height, and sarcopenia was defined by sex-specific cutoff values in several studies [41, 44]. However, TPA and cutoff values, calculated by these criteria, may show a certain difference between each study of populations with different age distribution and diseases. We defined sarcopenia as the lowest quartile with normalization for height and sex, resulting in an impressively high accuracy.
Our study had limitations. First, the number of patients with sarcopenia was small. Second, the perioperative abdominal CT scans were performed over a relatively long time (from 30 days prior to surgery to within 7 days of surgery). Third, this report is statistically limited because of the small number of patients, although sarcopenia was an independent risk factor in the multivariate analysis. Further prospective studies with larger numbers of patients and a CT protocol that is justified in terms of the scan timing are warranted.
In conclusion, sarcopenia, as measured by TPA obtained from cross-sectional imaging, is a risk factor for SSI in patients with UC undergoing surgery involving the creation of an IPAA. Further studies on larger numbers of patients and an adjusted CT protocol are necessary to confirm our findings.
References
Okita Y, Araki T, Kawamura M, Kondo S, Inoue M, Kobayashi M, et al. Clinical features and management of afferent limb syndrome after ileal pouch-anal anastomosis for ulcerative colitis. Surg Today. 2016. doi:10.1007/s00595-016-1307-7.
Miki C, Ohmori Y, Yoshiyama S, Toiyama Y, Araki T, Uchida K, et al. Factors predicting postoperative infectious complications and early induction of inflammatory mediators in ulcerative colitis patients. World J Surg. 2007;31:522–9 (discussion 30–1).
Kimura H, Takahashi K, Futami K, Ikeuchi H, Tatsumi K, Watanabe K, et al. Has widespread use of biologic and immunosuppressant therapy for ulcerative colitis affected surgical trends? Results of a questionnaire survey of surgical institutions in Japan. Surg Today. 2015. doi:10.1007/s00595-015-1259-3.
Delgado-Rodriguez M, Gomez-Ortega A, Llorca J, Lecuona M, Dierssen T, Sillero-Arenas M, et al. Nosocomial infection, indices of intrinsic infection risk, and in-hospital mortality in general surgery. J Hosp Infect. 1999;41:203–11.
Astagneau P, Rioux C, Golliot F, Brucker G, Group INS. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect. 2001;48:267–74.
Kobayashi M, Mohri Y, Tonouchi H, Miki C, Nakai K, Kusunoki M, et al. Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surg Today. 2007;37:383–8.
Pendlimari R, Cima RR, Wolff BG, Pemberton JH, Huebner M. Diagnoses influence surgical site infections (SSI) in colorectal surgery: a must consideration for SSI reporting programs? J Am Coll Surg. 2012;214:574–80 (discussion 80–1).
Araki T, Okita Y, Uchino M, Ikeuchi H, Sasaki I, Funayama Y, et al. Risk factors for surgical site infection in Japanese patients with ulcerative colitis: a multicenter prospective study. Surg Today. 2014;44:1072–8.
Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–30.
Sagar PM, Pemberton JH. Intraoperative, postoperative and reoperative problems with ileoanal pouches. Br J Surg. 2012;99:454–68.
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for disease control and prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;1999(27):97–132 (quiz 3–4, discussion 96).
Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4:5.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123:727–8.
Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96.
Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–7.
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–6.
Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108:1034–41.
Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65.
Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pract. 2012;27:593–8.
Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–86.
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–6.
Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13:439–46.
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22:2663–8.
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewe KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–52.
Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–402.
Onodera T, Goseki N. Kosaki G [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001–5.
Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–61.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Uchino M, Ikeuchi H, Matsuoka H, Tsuchida T, Tomita N, Takesue Y. Risk factors associated with surgical site infection after ileal pouch-anal anastomosis in ulcerative colitis. Dis Colon Rectum. 2010;53:143–9.
Madbouly KM, Senagore AJ, Remzi FH, Delaney CP, Waters J, Fazio VW. Perioperative blood transfusions increase infectious complications after ileoanal pouch procedures (IPAA). Int J Colorectal Dis. 2006;21:807–13.
Kiely JM, Fazio VW, Remzi FH, Shen B, Kiran RP. Pelvic sepsis after IPAA adversely affects function of the pouch and quality of life. Dis Colon Rectum. 2012;55:387–92.
Ziv Y, Fazio VW, Church JM, Lavery IC, King TM, Ambrosetti P. Stapled ileal pouch anal anastomoses are safer than handsewn anastomoses in patients with ulcerative colitis. Am J Surg. 1996;171:320–3.
Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2014;261:1173–83.
Hrnciarikova D, Juraskova B, Zadak Z, Hronek M. Present state of evaluating malnutrition in the elderly–analysing indicators. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:217–21.
Reijnierse EM, Trappenburg MC, Leter MJ, Sipila S, Stenroth L, Narici MV, et al. Serum albumin and muscle measures in a cohort of healthy young and old participants. Age (Dordr). 2015;37:88.
Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(526):e9–17.
Reisinger KW, Derikx JP, van Vugt JL, Von Meyenfeldt MF, Hulsewe KW, Olde Damink SW, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2015. doi:10.1016/j.clnu.2015.07.005.
Bastiaanse LP, Hilgenkamp TI, Echteld MA, Evenhuis HM. Prevalence and associated factors of sarcopenia in older adults with intellectual disabilities. Res Dev Disabil. 2012;33:2004–12.
Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbecks Arch Surg. 2014;399:287–95.
Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3:269–75.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 1985;2004(97):2333–8.
Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol. 2015;112:503–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Hiroyuki Fujikawa, Toshimitsu Araki, Yoshiki Okita, Satoru Kondo, Mikio Kawamura, Junichiro Hiro, Yuji Toiyama, Minako Kobayashi, Koji Tanaka, Yasuhiro Inoue, Yasuhiko Mohri, Keiichi Uchida, and Masato Kusunoki declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Fujikawa, H., Araki, T., Okita, Y. et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today 47, 92–98 (2017). https://doi.org/10.1007/s00595-016-1357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-016-1357-x