Abstract
Purpose
Molecular markers as indicators for gastric cancer recurrence are urgently required. The aim of this study was to identify lectins that can be used to predict gastric cancer recurrence after gastrectomy.
Methods
We created lectin expression profiles by microarray analysis for 60 patients, who underwent surgery for gastric cancer at the Oita University Hospital between January, 2005 and December, 2007. Lectin expression and clinicopathological factors in patients who suffered gastric cancer recurrence and those who did not were compared by univariate and multivariate analyses.
Results
Thirteen lectins showed a significant increase in binding to cancer tissues, whereas 11 lectins showed a significant decrease in binding to cancer tissues, when compared with binding to normal epithelia. Multivariate analysis revealed that lymph node metastasis and low Bauhinia purpurea lectin (BPL)-binding signals were independent predictive factors for recurrence. All patients with low BPL expression had significantly worse relapse-free survival than those with high BPL expression.
Conclusions
Our results using a novel lectin microarray system provide the first solid evidence that BPL expression is a predictor of gastric cancer recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common malignancy and the second leading cause of cancer-related death worldwide [1]. While the survival rates of patients with gastric cancer have increased steadily with improvements in early detection and surgical techniques, clinical outcomes remain unfavorable for patients with advanced disease. Adjuvant chemotherapy is often administered after complete tumor resection (R0) to improve long-term survival in this group of patients [2–4]. Since recurrence can develop even in patients with early stage gastric cancer, it is very important to identify markers that predict gastric cancer recurrence after curative surgery.
Several reports have described various clinicopathological factors, such as tumor invasion, lymph node status, lymphatic invasion, venous invasion, and neutrophil/lymphocyte ratio (NRL), as predictors of recurrence and prognosis [5–8]. Carcinoembryonic (CEA) and carbohydrate antigen 19-9 (CA19-9) are glycoproteins, and altered cancer glycan profiles have been used clinically to detect recurrence or metastasis [9]. Human epidermal growth factor receptor 2 (HER2) [10], epidermal growth factor receptor (EGFR) [11], E-cadherin [12], cluster of differentiation 44 (CD44) [13], and cytokeratin 20 [14] are of little clinical predictive value in patients with gastric cancer; therefore, additional research has been directed towards identifying novel predictive factors.
Altered glycosylation patterns in cells and specific glycosyltransferases are linked to carcinogenesis, differentiation, proliferation, and adhesion [15]. Although these alterations have been observed in human breast [16], prostate [17], thyroid [18], and ovarian cancers [19], few clinically useful predictors are known for gastric cancer. Glycan profiles have been established in various cancers by using lectin microarray systems [20–22]. We previously analyzed glycan profiles in resected colon cancer specimens by lectin microarray, a new approach to discover biologically relevant glycosylation changes between normal and tumor tissues using a very small piece of tissue [21]. Furthermore, we identified the key lectins involved in distant recurrence of colon cancer [23]. Based on our findings, we propose that lectin microarray profiling is a promising method to detect target lectins related to cancer aggressiveness. The aim of this study was to identify predictors of gastric cancer recurrence using the novel lectin microarray system.
Materials and methods
Patients
We collected surgical specimens from and accessed the medical records of 60 patients who underwent curative resection of gastric cancer at the Department of Gastroenterological and Pediatric Surgery, Oita University Faculty of Medicine, between January, 2005 and December, 2007. All cancers were classified using the International Union against Cancer-TNM staging system 7 [24]. Adjuvant chemotherapy was given to patients with pathological stage II or III (without T1 or T3/N0) cancer, according to the Japanese gastric cancer treatment guidelines. Patients who received preoperative chemotherapy were excluded from the analysis. All patients were followed up every 6 months by computed tomography. We obtained written informed consent from all patients.
Sample preparation
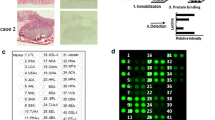
Formalin-fixed, paraffin-embedded Sects. (10-μm thick for the lectin microarray and 3-μm thick for lectin staining) of gastric cancer tissue and normal epithelium from the same clinical specimen were placed on glass slides and deparaffinized. After hematoxylin and eosin staining, the areas of malignant cells were marked for macrodissection using a magnifying glass. A region including normal epithelial tissue was also macrodissected.
Lectin microarray
The lectin microarray was performed according to the manufacturer’s instructions, as described previously [23]. Briefly, the samples were washed three times with phosphate-buffered saline (PBS), tissue pellets were collected by centrifugation, solubilized in 20 μL PBS containing 0.5 % Nonidet P 40, and sonicated using a Bioruptor UCW-310 (Cosmo Bio, Carlsbad, CA, USA). Protein concentrations were quantified using the Micro BCA Protein Assay Reagent Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) and diluted to 15 μg/mL with PBS. The glycoprotein fractions were labeled with cyanine 3 fluorescent dye, adjusted to 250 ng/mL using Probing Solution (GP Biosciences Ltd., Yokohama, Japan), and applied to each well of the LecChip™ (GP Biosciences Ltd.). Furthermore, the fractions were incubated in the dark (>80 % humidity at 20 °C) for 15 h. The LecChip™ wells were washed three times with Probing Solution before application, to decrease background. After the incubation, fluorescent images of the lectin arrays were obtained using an evanescent-field fluorescence scanner GlycoStation™ Reader 1200 (GP Biosciences Ltd.), and the data were analyzed with GlycoStation™ Tool Pro Suite 1.5. Lectin–glycan interaction values were normalized for more than 45 different lectins by setting the average of 45 lectin intensities to 100.
Immunohistochemistry of BPL expression in normal and cancerous gastric tissues
Immunohistochemistry was performed according to a standard protocol [23, 25]. Biotinylated BPL (Vector Laboratories, Burlingame, CA, USA) was used as the primary antibody and the VECTASTAIN® Elite ABC Reagent (Vector Laboratories) was used as the secondary antibody.
Statistical analysis
Spot intensity values were calculated by subtracting the background value from the raw signal intensity value. These lectin signal intensity values, obtained from normal tissue (n = 60) and cancer tissue (n = 60), were normalized and averaged, and the ratios of tumor vs. normal (T/N) were calculated as previously described [21]. Differences in lectin signals between the tumor and normal tissue groups were analyzed by the Mann–Whitney test. Strong correlations (R 2 = 0.999) were observed between the normalized and non-normalized data sets. Differences in prognostic factors between patient subgroups were analyzed with the Chi-square test. The Kaplan–Meier method was used for survival analysis and statistical significance was determined using the log-rank test. A p value of <0.05 was considered significant. Multivariate survival analyses were performed using the logistic regression. The lectin cutoff point was determined using a receiver operating characteristic (ROC) curve. All analyses were performed using SPSS ver. 20 (SPSS Inc., Chicago, IL, USA).
Results
Identification of lectins differentially expressed in gastric cancer tissue compared with those in normal tissue
The expression levels of 45 lectins in gastric cancer and normal tissues from resected specimens were analyzed using a lectin microarray (Table 1). The expression of 13 lectins, including LCA, MAL-I, SNA, SSA, TJA-I, NPA, ConA, GNA, HHL, ACG, TxLC-I, Jacalin, and MAH, was increased significantly in cancer tissue compared with that in normal tissue. In contrast, the expression of 11 lectins, including UEA-I, AOL, AAL, BPL, EEL, ABA, WFA, ACA, DBA, SBA, and GSL-I-A4, was decreased. There were no significant differences in the other lectins between the cancer and normal tissues.
Association of clinicopathological factors, expression of lectins, and gastric cancer recurrence in univariate and multivariate analyses
Recurrence developed in 19 patients, as distant lymph node metastasis in 9, peritoneal recurrence in 7, and liver, lung, and local recurrence in 1 patient each. We divided the patients into two groups: one comprised of 41 patients without recurrence and one comprised of 19 patients with recurrence after gastrectomy. There were no significant differences between the groups in age or sex in the univariate analysis (Table 2). No correlation was observed between recurrence and adjuvant chemotherapy in patients with pathological Stages II and III (without T1 or T3/N0) cancer. In contrast, there were significant differences between the groups in histology (p = 0.020), depth of invasion (p = 0.003), lymph node metastasis (p < 0.001), lymphatic invasion (p = 0.007), venous invasion (p = 0.034), and pathological stage (p < 0.001). After the cutoff point was established using a ROC curve between the groups, each lectin was categorized into a high or low expression group using the cutoff point. In a univariate analysis, significant associations were observed for the BPL (p = 0.016), EEL (p = 0.021), and GSL-I-A4 (p = 0.005) binding signals (T/N ratio) between patients with and those without gastric cancer recurrence. There were no significant differences between the groups for the other lectins. Factors showing a strong correlation in the univariate analysis were selected for multivariate analysis. The multivariate analysis included histology, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, and three lectins; anamely, BPL, EEL, and GSL-I-A4. The multivariate analysis revealed that lymph node metastasis [p = 0.004; relative risk (RR), 0.092; 95 % confidence interval (CI), 0.018–0.465] and BPL expression level (p = 0.039; RR, 5.310; 95 % CI, 1.086–25.95) were independent predictors of recurrence after primary gastric cancer resection; however, the expression of BPL was not associated with any recurrence pattern.
Correlation between relapse-free survival and BPL expression
The median follow-up time after surgery was 68 months (range, 6–84 months). In all cases, the Kaplan–Meier survival curves indicated that patients with low BPL levels had significantly worse relapse-free survival (p = 0.006) than those with high BPL levels (Fig. 1a). Since adjuvant chemotherapy is not recommended for patients with pathological T1-3 and N0 according to the Japanese gastric cancer treatment guidelines, we focused only on those patients to find new candidates for adjuvant chemotherapy. As a result, among patients with pathological T1-3 and N0 disease, those with low BPL levels had significantly worse relapse-free survival (p = 0.031) than those with high BPL levels (Fig. 1b).
Relapse-free survival in relation to high vs. low Bauhinia purpurea lectin (BPL) levels. The Kaplan–Meier survival curves in all cases indicated that patients with low BPL levels had significantly worse relapse-free survival (p = 0.006) than those with high BPL levels (a). Among patients with pathological T1-3 and N0 disease, those with low BPL levels had significantly worse relapse-free survival (p = 0.031) than those with high BPL levels (b)
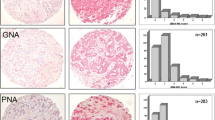
Lectin staining
The distribution of BPL expression differed between normal and cancerous tissues (Fig. 2). BPL expression was detected in the cytoplasm and mucin of normal tissue, whereas it was detected in the cell membranes of cancer tissue.
Discussion
Our results demonstrated that BPL expression was a predictor of gastric cancer recurrence. Therefore, if this marker can identify the high-risk group for gastric cancer recurrence, it would contribute to improving the prognosis of patients with gastric cancer. Our results provide the first documented evidence that a specific lectin is a predictor of gastric cancer recurrence, using a novel lectin microarray system.
In the present study, glycan expression was analyzed using a lectin microarray method. Capillary electrophoresis and liquid chromatography techniques are commonly used to evaluate glycans, although there are several limitations to these methods: they require a heterogeneous preparation, they have low sensitivity and low efficiency, and they are time consuming [26]. We used a lectin microarray to exhaustively determine the glycan expression profiles of 45 lectins using one glass slide of formalin-fixed clinical samples [20–22]. There are several advantages to this lectin profiling system, including simple preprocessing, high sensitivity, reproducibility, throughput, and performance.
We identified BPL as a significant lectin in gastric cancer recurrence. BPL is a plant lectin isolated from leguminous seeds, which agglutinates human asialo erythrocytes regardless of their ABO blood group type [27]. It is a protein containing a long metal-binding loop, part of which determines its carbohydrate specificity [28]. It has been reported that BPL expression in colon cancer tissue is remarkably lower than that in normal tissue [21]. N-acetyl-galactosamine, which is specific to BPL [29], is an essential structure in mucin produced in goblet cells. Thus, the decreased expression of BPL in cancer tissue observed here may have been caused by a dysfunction in mucin production. Shiratsu et al. reported that loss of a mucin-specific glycan is associated with poor prognosis and disease progression in patients with gastric cancer [30]. Karasawa et al. [31] reported that a mucin-specific glycan functions as a tumor suppressor in gastric adenocarcinoma, and that its loss triggers gastric carcinogenesis through inflammation-associated pathways. Previous reports revealed that inflammation influences every step of tumorigenesis from initiation to metastatic progression [32–34]. Accordingly, decreased BPL expression may be associated with tumor progression and poor prognosis in patients with gastric cancer.
The current study revealed that BPL staining occurred exclusively in the cytoplasm of goblet cells and that mucin was present in normal tissue. In contrast, BPL staining occurred specifically in the cell membrane of cancer tissue. This difference in BPL location suggests a change in glycogen structure, which may be reflected during carcinogenesis. Decreased expression of BPL would be related to metastasis and recurrence of gastric cancer.
Studying CEA and CA19-9, which are glycoproteins with altered glycan profiles in various cancers, is useful during monitoring after gastric cancer surgery [35–38]. However, these tumor markers are not reliable predictors of recurrence at the time of initial gastrectomy. Conversely, we consider that BPL expression is a useful predictor of gastric cancer recurrence just at the time of curative resection, based on the results of the lectin microarray analysis using resected specimens.
Some previous studies demonstrated that several factors such as histopathological type, tumor depth, tumor size, lymphatic and venous invasion were significantly associated with gastric cancer recurrence [5–7, 39–42]. According to the multivariate analysis in our study, BPL expression was of significantly higher value than those factors. Moreover, we found a significant difference in relapse-free survival between patients with pathological T1-3and N0 disease with high and low BPL levels. Therefore, we demonstrated that BPL expression could also be an indicator of candidates for adjuvant chemotherapy.
This study had several limitations. Certain factors, such as sample location, the presence of gastritis or diabetes mellitus, and whether or not the patient was taking an oral proton pump inhibitor, may have affected the epithelial glycan structure. Further studies on the effects of these conditions on lectin profiling are required. Moreover, considering the relatively small number of patients, it will be necessary to evaluate the clinical utility of BPL expression using a larger cohort. We expect that the new BPL biomarker will contribute to improving the long-term clinical outcomes of patients with gastric cancer.
Conclusion
Using a novel lectin microarray system, we identified that BPL expression was a predictor of gastric cancer recurrence.
Abbreviations
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- HER2:
-
Human epidermal growth factor receptor 2
- EGFR:
-
Epidermal growth factor receptor
- CD44:
-
Cluster of differentiation 44
- ROC:
-
Receiver operating characteristic
- LCA:
-
Lens culinaris agglutinin
- MAL-I:
-
Maackia amurensis lectin I
- SNA:
-
Sambucus nigra agglutinin
- SSA:
-
Sambucus sieboldiana agglutinin
- TJA-I:
-
Trichosanthes japonica agglutinin I
- NPA:
-
Narcissus pseudonarcissus lectin
- ConA:
-
Concanavalin A
- GNA:
-
Galanthus nivalis agglutinin
- HHL:
-
Hippeastrum hybrid lectin
- ACG:
-
Agrocybe cylindracea galectin
- TxLC-I:
-
Tulipa gesneriana I
- MAH:
-
Maackia amurensis hemagglutinin
- UEA-I:
-
Ulex europaeus agglutinin I
- AOL:
-
Aspergillus oryzae lectin
- AAL:
-
Aleuria aurantia lectin
- BPL:
-
Bauhinia purpurea lectin
- EEL:
-
Euonymus europaeus lectin
- ABA:
-
Agaricus bisporus agglutinin
- WFA:
-
Wisteria floribunda lectin
- ACA:
-
Amaranthus caudatus agglutinin
- DBA:
-
Dolichos biflorus agglutinin
- SBA:
-
Soybean agglutinin
- GSL-I-A4:
-
Griffonia simplicifolia
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Bang Y-J, Kim Y-W, Yang H-K, Chung HC, Park Y-K, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93.
Msika S, Benhamiche A, Jouve J-L, Rat P, Faivre J. Prognostic factors after curative resection of gastric cancer: a population-based study. Eur J Cancer. 2000;36:390–6.
Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64–73.
Kunisaki C, Makino H, Kimura J, Takagawa R, Kosaka T, Ono HA, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147:204–11.
Nakayama Y, Gotohda N, Shibasaki H, Nomura S, Kinoshita T, Hayashi R. Usefulness of the neutron/lymphocyte ratio measured preoperatively as a predictor of peritoneal metastasis in patients with advanced gastric cancer. Surg Today. 2014;44:2146–52.
Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6:142–5.
Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer. 2012;3:137–44.
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Shimada Y, Yamasaki S, Hashimoto Y, Ito T, Kawamura J, Soma T, et al. Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res. 2004;10:2818–23.
Yamamichi K, Uehara Y, Kitamura N, Nakane Y, Hioki K. Increased expression of CD44v6 mRNA significantly correlates with distant metastasis and poor prognosis in gastric cancer. Int J Cancer (Pred Oncol). 1998;79:256–62.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142–8.
Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130.
Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–69.
Lange T, Ullrich S, Muller I, Nentwich MF, Stubke K, Feldhaus S, et al. Human prostate cancer in a clinically relevant xenograft mouse model: identification of beta(1,6)-branched oligosaccharides as a marker of tumor progression. Clin Cancer Res. 2012;18:1364–73.
Chen G, Wang Y, Qiu L, Qiu X, Liu H, Wang X, et al. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteomics. 2012;75:2824–34.
Saldova R, Dempsey E, Perez-Garay M, Marino K, Watson JA, Blanco-Fernandez A, et al. 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 2011;6:1362–72.
Fry SA, Afrough B, Lomax-Browne HJ, Timms JF, Velentzis LS, Leathem AJ. Lectin microarray profiling of metastatic breast cancers. Glycobiology. 2011;21:1060–70.
Matsuda A, Kuno A, Ishida H, Kawamoto T, Shoda J, Hirabayashi J. Development of an all-in-one technology for glycan profiling targeting formalin-embedded tissue sections. Biochem Biophys Res Commun. 2008;370:259–63.
Nishijima Y, Toyoda M, Yamazaki-Inoue M, Sugiyama T, Miyazawa M, Muramatsu T, et al. Glycan profiling of endometrial cancers using lectin microarray. Genes Cells. 2012;17:826–36.
Nakajima T, Inomata M, Iha H, Hiratsuka T, Etoh T, Shiraishi N, et al (2015) Establishment of new predictive markers for distant recurrence of colorectal cancer using lectin microarray analysis. Cancer Medicine (in press).
Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumors, 7th edition, Oxford. UK: Wiley-Blackwell; 2009.
Huang W-L, Li Y-G, Lv Y-C, Guan X-H, Ji H-F, Chi B-R. Use of lectin microarray to differentiate gastric cancer from gastric ulcer. World J Gastroenterol. 2014;20(18):5474–82.
Vanderschaeghe D, Festjens N, Delanghe J, Callewaert N. Glycome profiling using modern glycomics technology: technical aspects and applications. Biol Chem. 2010;391:149–61.
Wu AM, Kabat EA, Gruezo FG, Allen HJ. Immunochemical studies on the combining site of the D-Galactopyranose and 2-Acetamid-2-deoxy-D-Galactopyranose specific lectin isolated from Bauhinia purpurea alba seeds. Arch Biochem Biophys. 1980;204:622–39.
Yamamoto K, Maruyama IN, Osawa T. Cybrog lectins: novel leguminous lectins with unique specificities. J Biochem. 2000;127:137–42.
Iramura T, Osawa T. Studies on a hemagglutinin from Bauhinia purpurea alba seeds. Arch Biochem Biophys. 1972;151:475–81.
Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Sci. 2014;105:126–33.
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, et al. Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest. 2012;122:923–34.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6.
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKb and JNK1 dependent inflammation. Cancer Cell. 2010;17(1):89–97.
Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–99.
Ohkura H. Tumor markers in monitoring response to chemotherapy for patients with gastric cancer. Jpn J Clin Oncol. 1999;29:525–6.
Yamao T, Kai S, Kazami A, Koizumi K, Handa T, Takemoto N, Maruyama M. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn J Clin Oncol. 1999;29:550–5.
Pectasides D, Mylonakis A, Kostopoulou M, Papadopoulou M, Triantafillis D, Varthalitis J, et al. CEA, CA 19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol. 1997;20:348–53.
Kodama I, Koufuji K, Kawabata S, Tetsu S, Tsuji Y, Takeda J, Kakegawa T. The clinical efficacy of CA 72-4 as serum marker for gastric cancer in comparison with CA19-9 and CEA. Int Surg. 1995;80:45–8.
Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;89(7):1418–24.
Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, Noh SH. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology. 2009;77(3–4):197–204.
Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252(1):70–3.
Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4(2):137–40.
Acknowledgments
This study was supported, in part, by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (24591953). We thank Ms. Mayumi Takeda, Ms. Hiroko Taguchi, Ms. Yuiko Aso, and Ms. Maki Fujinaga for technical assistance.
Conflict of interest
T. Futsukaichi and his coauthors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Futsukaichi, T., Etoh, T., Nakajima, K. et al. Decreased expression of Bauhinia purpurea lectin is a predictor of gastric cancer recurrence. Surg Today 45, 1299–1306 (2015). https://doi.org/10.1007/s00595-015-1127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1127-1