Abstract
Purpose
The aim of this study was to retrospectively assess the oncological safety of breast-conserving surgery (BCS) after primary systemic chemotherapy (PST) in terms of local recurrence (LR) in cT3–4 patients.
Methods
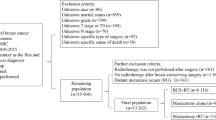
The subjects were 146 cT1–2 patients who underwent BCS after PST, and 169 patients with cT3–4 primary breast cancer. Of the 169 patients with cT3–4 disease, 20 underwent surgery first, and 149 underwent surgery after PST (mastectomy: 101 patients; BCS: 48 patients). The LR-free survival (LRFS) was analyzed using a Kaplan–Meier analysis. We evaluated the predictors using Cox proportional hazards modeling for LR after PST.
Results
There was no significant difference in 5-year LRFS between the cT1–2 and cT3–4 groups that underwent BCS after PST (98.6 vs. 92.5 %; P = 0.074). The 5-year LRFS was 94.7 % in the group that underwent initial surgery and 93.0 % in the PST group (P = 0.845) in the cT3–4 patients, while the 5-year LRFS rates were 93.2 % in the BCS subgroup and 92.5 % in the mastectomy subgroup (P = 0.958). In a multivariate analysis, the histological type, hormone negativity and a higher histological grade were independent predictors of LR after PST.
Conclusions
BCS after PST may be oncologically acceptable for cT3–4 breast cancers in terms of the LR compared with initial surgery or mastectomy after PST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast-conserving surgery (BCS) has been extensively used for breast cancer management. It provides the same curability as mastectomy, and it leads to good cosmetic outcomes. In recent years, endoscopic BCS has also been reported, which provides better cosmetic results than conventional direct vision surgery [1]. The indications for BCS have been further expanded because of the introduction of primary systemic chemotherapy (PST), and BCS is sometimes used for some locally advanced breast cancers, even for tumors >5 cm (cT3) or extending to the chest wall or skin (cT4).
PST was first used in the early 1970s to treat non-operable breast cancer (locally advanced or inflammatory). Its use was gradually extended to operable large breast tumors (T > 5 cm; ~1980s) and operable small breast tumors (T > 1–2 cm; ~1990s) [2]. The initial aim of PST was to achieve operability for locally advanced breast cancer, and then gradually improve the breast conservation rates, by testing the in vivo tumor chemosensitivity. Randomized studies with long follow-up periods comparing primary vs. adjuvant chemotherapy found no differences in the disease-free and overall survival rates, but showed that primary chemotherapy led to more frequent BSC [3–8]. More recently, PST has been used to treat operable locally advanced or large primary tumors to increase the rate of conservative surgery. PST is now considered the standard of care for locally advanced breast cancer, a reasonable option for large primary breast tumors not eligible for conservative surgery and an acceptable alternative for all patients who are candidates for adjuvant treatment. However, some barriers to BCS after PST exist.
For example, the presence of a residual tumor after PST is considered to be a risk factor for local recurrence (LR) [9, 10], and is an obstacle to obtaining clear resection margins when attempting BCS. In addition, a delay of surgery in patients who show resistance to PST allows the disease to progress. Under these circumstances, the use of BCS for patients with locally advanced tumors downstaged by PST remains controversial. In other words, the oncological safety of BCS after PST for patients with initial large tumors (>5 cm; cT3) or tumors with direct extensions to the chest wall or skin (cT4) is not thoroughly established. This study evaluated the oncological safety of BCS after PST in cT3–4 patients in terms of the LR.
Materials and methods
The subjects were 146 patients with cT1–2 primary breast cancer who underwent BCS after PST, and 169 consecutive patients with cT3–4 primary breast cancers who underwent surgery between January 2004 and October 2011 at the National Cancer Center Hospital, Tokyo, Japan. In this study, LR was defined as the first recurrent event that occurred in the remaining breast (in cases of BCS) or in the chest wall or chest skin (in cases that underwent mastectomy).
First, we compared the LR rates between the cT1–2 group and the cT3–4 group that underwent BCS after PST. Next, we subdivided the cT3–4 group into two subgroups: the initial surgery (IS) group and the PST group. Thereafter, we subdivided the PST group into two subgroups: the BCS subgroup and the mastectomy subgroup. The LR-free survival in each group was analyzed using a Kaplan–Meier analysis, and we evaluated predictors by means of Cox proportional hazards modeling for the LR in patients after PST. All patients in the IS group underwent mastectomy. In the PST group, patients with tumors ≤3 cm after PST were eligible for BCS, but some with a tumor size >3 cm were included after PST if the breast had a cosmetic deformity or defect that could be corrected surgically. When performing BCS after PST, we determined the extent of resection according to both the location and spread of the tumor after PST. The remaining tumor was resected with a 1- to 3-cm margin from the suspected range observed in the imaging results/physical findings after PST. In addition, the patients who received a diagnosis of a non-preservable nipple underwent mastectomy. Patients with inflammatory and metastatic tumors were excluded from this study. In the PST group, all patients underwent axillary dissection. In the IS group, all patients underwent axillary dissection, except for two patients who were clinically node negative and underwent sentinel lymph node biopsy based on the surgeon’s discretion.
The clinical and pathological T and N factors were based on the Cancer Staging Manual of the American Joint Committee on Cancer (AJCC), 7th edition. Of the 146 cT1–2 patients, two (1.4 %) had a cT1 tumor and 144 (98.6 %) had a cT2 tumor. Of the 169 cT3–4 patients, 126 (75 %) had a cT3 tumor and 43 (25 %) had a cT4 tumor. The cutoff values for the estrogen receptor (ER) and progesterone receptor (PgR) positivity were 1 % positive cells for both, irrespective of the intensity. Human epidermal growth factor receptor 2 (HER2) positivity was defined as a HER2 score >2 (>30 % strong membrane immunoreaction-positive cells) or a HER2 gene/centromere-17 ratio ≥ 2.0, as assessed by fluorescence in situ hybridization. The ER, PgR and HER2 status were examined both before and after PST by more than two pathologists. The margin status was classified as positive or negative. Positive margins were defined as margins involved by invasive or non-invasive tumor; negative margins were defined as those free from invasive or non-invasive tumors.
The PST protocol and treatment of adverse effects were managed by clinical oncologists. Of the 149 patients, 143 underwent treatment with both anthracycline (A) and taxane (T)-containing regimens (A + T), four patients were treated with an A regimen only, and two patients were treated with a T regimen only. The A + T regimen consisted of four cycles of doxorubicin (60 mg/m2) plus cyclophosphamide (600 mg/m2), followed by weekly paclitaxel (80 mg/m2), four cycles of epirubicin (100 mg/m2), cyclophosphamide (500 mg/m2) and 5-fluorouracil (500 mg/m2), followed by weekly paclitaxel (80 mg/m2), and four cycles of concurrent doxorubicin (50 mg/m2) plus docetaxel (50 mg/m2). Radiological examinations by MRI or CT and US for evaluation of the clinical response were performed at both before and after PST for every patient. Pathologists also recorded the pathologically invasive tumor size, pathological nodal status, histological grade, the presence or absence of lymphovascular invasion and the pathologic response to PST based on the findings in each surgical specimen. The definition of a pathological complete response (pCR) allowed for residual cancer in the intraductal component. Non-pCR was defined as the presence of residual tumor in the affected breast on permanent surgical specimens.
Patients who underwent BCS received radiation therapy for the entire breast (50–60 Gy). Axillary and supraclavicular radiation was performed on patients with a clinical N2 or higher pathological N stage. Patients who underwent mastectomy also received radiation therapy to the chest wall, axillary and supraclavicular regions (50–60 Gy) if the tumor was clinically N2 stage or higher. The length of follow-up was measured from the date of surgery.
Statistical analysis
We used the Mann–Whitney U test to compare the ages between the patient subsets, the χ 2 test to compare other variables and a Cox regression analysis to investigate the hazard ratios (HR) of individual parameters for predicting the LR. A value of P < 0.05 was considered to be statistically significant. Confidence intervals (CIs) were set at 95 %. The SPSS statistical software program (version 19, IBM SPSS Statistics, Chicago, IL, USA) was used for all statistical analyses.
Results
Table 1 summarizes the clinicopathological parameters of the cT1–2 group compared to the cT3–4 group after PST. There were no significant differences in the clinical parameters between the two groups except for the cT stage. LR developed in three cases in the cT3–4 group, and in two cases in the cT1–2 group (P = 0.064). HER2 positivity tended to be higher in the LR group compared with that in the patients without LR (P = 0.020). The 5-year LRFS rates were 92.5 % in the cT3–4 group and 98.6 % in the cT1–2 group (P = 0.074; Fig. 1).
Table 2 summarizes the clinicopathological parameters of the IS group compared to the PST group. The age, cT stage and use of radiation therapy significantly differed between the two groups (P = 0.016, P = 0.025, P < 0.001, respectively). In terms of PST, a higher histological grade (G3 to G1–2), hormone negativity, cCR and pCR were observed more frequently in the BCS subgroup (P = 0.030, P = 0.024, P = 0.015, and P = 0.002, respectively), whereas the cT stage, pN stage and HER2 negativity were higher in the mastectomy subgroup (P = 0.011, P = 0.010, and P = 0.027, respectively). At a median follow-up of 61 months (range 1–116), LR had developed in one patient in the IS group, and in nine patients in the PST group. The 5-year LR-free survival rates were 94.7 % in the IS group and 93.0 % in the PST group, and did not significantly differ between the two groups (P = 0.845; Fig. 2a). The 5-year overall survival rates also did not significantly differ between the groups (P = 0.507; Fig. 2b).
LR developed in three patients in the BCS subgroup and six patients in the mastectomy subgroup. The 5-year LR-free survival rates were 93.2 and 92.5 %, respectively, and did not differ significantly (P = 0.958; Fig. 3a). Similarly, the 5-year overall survival rates also did not differ significantly (P = 0.568; Fig. 3b).
The associations of the clinicopathological characteristics with the LR were analyzed in a multivariate Cox regression model in the overall cT3–4 patients. Lymphovascular invasion (present vs. absent: HR, 4.720; P = 0.044), hormone receptor status (negative vs. positive: HR, 5.921; P = 0.033) and a higher histological grade (grade 3 vs. grades 1–2: HR, 17.862; P = 0.024) were identified as independent predictors for the LR in the overall cT3–4 patients (Table 3). Similarly, the histological type (special type vs. invasive ductal: HR, 15.595; P = 0.024), hormone receptor status (negative vs. positive: HR, 7.079; P = 0.042) and a higher histological grade (grade 3 vs. grade 1–2: HR, 41.773; P = 0.013) were identified as independent predictors of the LR in PST cases (Table 4).
Discussion
The oncological safety of BCS after PST in patients with larger breast tumors (cT3–4) compared with that of patients with small tumors (cT1–2) has not been thoroughly established. Up to now, the initial tumor size and quality of the clinical response were thought to correlate with the rate of success after BCS. In the B-18 trial, patients who received PST improved their chance for breast conservation by 12.5 % if their tumor was 2–5 cm, and by 17.5 % if the tumor was >5 cm [4]. Rouzier et al. [11] developed a nomogram to predict the probability of a patient becoming eligible for BCS after PST. The factors used in this nomogram included the ER status, initial tumor size, histological grade, multicentricity and histological type. In addition, the GEPARDUO trial suggested that the factors associated with a significantly higher BCS rate were a pre-chemotherapy tumor size ≤40 mm, non-lobular histological characteristics, treatment with an adriamycin, cyclophosphamide and docetaxel regimen, the clinical response and a post-chemotherapy tumor size ≤20 mm [12]. These data indicated that both the initial and post-chemotherapy primary tumor sizes are essential for determining the eligibility for BCS.
On the other hand, a retrospective review of 403 cases who underwent BCS after PST at M.D. Anderson Cancer Center identified four clinicopathological factors as predictors of LR for BCS after PST in a multivariate analysis: cN2–3 stage, presence of lymphovascular invasion, residual pathological tumor size >2 cm and a multifocal residual pattern of disease, whereas the initial T stage (T1–2 vs. T3–4) did not correlate with the LR (5-year ipsilateral breast tumor recurrence-free rates of 96 vs. 92 %, respectively, P = 0.19) [9]. This study found that BCS after PST had an acceptably low rate of LR in selected patients, even those with cT3–4 patients. Similarly, although some papers have reported that the clinical stage and quality of clinical and pathological response correlated with the LR rate in patients who underwent BCS after PST, the initial tumor size did not correlate with LR in these patients [10, 13–18]. Although these results imply that the initial tumor size is not a risk factor for LR, the post-chemotherapeutic response with regard to the tumor-related factors and biological features of the tumor itself do influence LR. Furthermore, Rouzier et al. [13] concluded that LR in downstaged patients was a strong independent predictor of distant metastasis, and reported that the initial tumor size did not correlate with distant metastasis.
In this study, we first compared the LR rates between the cT3–4 and cT1–2 patients who underwent BCS after PST. Despite a tendency for LR to develop more often in the cT3–4 group, a significant difference was not observed between the two groups. Next, we compared the LR rates of cT3–4 breast cancer patients in the PST and the IS groups, and again found no significant differences in the LRFS between the two groups. The two groups did not differ significantly in the period of follow-up, cN stage, histological type, lymphovascular invasion, margin status, histological grade, pN stage, hormone status or HER2 status. However, the number of patients who underwent radiation therapy was higher in the PST group, apparently because patients who underwent BCS routinely received radiotherapy. The age and cT stage were higher in the IS group. Furthermore, we compared the LR rates of patients in the BCS and mastectomy subgroups, and found no significant difference in the LRFS between the two groups. The two groups did not significantly differ in the period of follow-up, cN stage, histological type, lymphovascular invasion, margin status or histological grade. However, the cT stage, histological grade, pN stage, hormone status, HER2 status, number of patients who underwent radiation therapy and clinical and pathological responses were significantly different between the two groups. The clinical and pathological CR rates were higher in the BCS subgroup than in the mastectomy subgroup in the PST cases, possibly because of the higher proportion of hormone negativity in the BCS subgroup, because hormone-negative breast cancer is reportedly more likely to exhibit a pathological CR [19], and because more downstaged patients were eventually included in the BCS subgroup.
However, the multivariate Cox regression analysis did not indicate that these post-chemotherapeutic response factors influenced the LR rate in the cT3–4 breast cancer patients in the present study. Instead, special breast cancer types, including metaplastic and invasive lobular breast cancers, hormone receptor negativity and a higher histological grade were associated with an increase in LR in the PST group. We believe that it is necessary to consider different treatment strategies for local recurrence (where a complete cure after treatment such as surgery may be possible) and against distant metastasis (where radical treatment cannot be expected to include surgical resection). Therefore, we believe that the local recurrence rate should be one of the major criteria for selecting BCS after PST against locally advanced cT3–4 tumors in the clinical setting.
In our study, the LR rate of the PST group was comparable to that of the IS group in cT3–4 patients after long-term follow-up. In the PST group, the BCS subgroup’s LR rate was also comparable to that of the mastectomy subgroup. In other words, these findings suggest that BCS after PST may be an alternative option to conventional surgery for cT3–4 breast cancer patients. In recent studies, some factors, including a younger age, pathological response, nodal status, lymphovascular invasion, cCR, higher histological grade, hormone receptor negative status and HER2 overexpression, were considered to be predictive factors for LR in the setting of PST [14, 16]. As expected, hormone receptor negative status and a higher histological grade were predictors of LR in our study, whereas a younger age, lymphovascular invasion, pCR and the pN status were not significantly associated with LR. We thought that this was probably due to our small sample size. In fact, LR did not occur in patients younger than 40 years, and this factor could not be analyzed in the multivariate analysis. The number of LR events was also small because of the small sample size of this study. If our sample size was larger, these factors might correlate with the LR. Moreover, this was a retrospective study, and its data might have been biased. Verification of the equivalence of the oncological safety would be possible only in a prospective non-inferiority study. Nonetheless, it appears that our results indicate the possibility of oncological safety of BCS for cT3–4 patients from the standpoint of a retrospective comparison of the LR. Larger clinical studies in this field are warranted.
In conclusion, despite some limitations of this study, our findings indicate that BCS after PST may be oncologically acceptable for cT3–4 breast cancers in terms of the LR. The biological characteristics, including the histological type, hormone receptor status and histological grade are predictors of the risk of LR in PST cases.
References
Takahashi H, Fujii T, Nakagawa S, Inoue Y, Akashi M, Toh U, et al. Usefulness of breast-conserving surgery for breast cancer. Surg Today. 2013;. doi:10.1007/s00595-013-0767-2.
Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21:2600–8.
Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999;10:47–52.
Broet P, Scholl SM, de la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat. 1999;58:151–6.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol. 1998;9:1179–84.
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–9.
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–12.
Ishitobi M, Ohsumi S, Inaji H, Ohno S, Shigematsu H, Akiyama F, et al. Ipsilateral breast tumor recurrence (IBTR) in patients with operable breast cancer who undergo breast-conserving treatment after receiving neoadjuvant chemotherapy: risk factors of IBTR and validation of the MD Anderson Prognostic Index. Cancer. 2012;118:4385–93.
Rouzier R, Pusztai L, Garbay JR, Delaloge S, Hunt KK, Hortobagyi GN, et al. Development and validation of nomograms for predicting residual tumor size and the probability of successful conservative surgery with neoadjuvant chemotherapy for breast cancer. Cancer. 2006;107:1459–66.
Loibl S, von Minckwitz G, Raab G, Blohmer JU, Dan Costa S, Gerber B, et al. Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Ann Surg Oncol. 2006;13:1434–42.
Rouzier R, Extra JM, Carton M, Falcou MC, Vincent-Salomon A, Fourquet A, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–35.
Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–6.
Miles RC, Gullerud RE, Lohse CM, Jakub JW, Degnim AC, Boughey JC. Local recurrence after breast-conserving surgery: multivariable analysis of risk factors and the impact of young age. Ann Surg Oncol. 2012;19:1153–9.
Tanioka M, Shimizu C, Yonemori K, Yoshimura K, Tamura K, Kouno T, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010;103:297–302.
Cebrecos I, Cordoba O, Deu J, Xercavins J, Rubio IT. Can we predict local recurrence in breast conserving surgery after neoadjuvant chemotherapy? Eur J Surg Oncol. 2010;36:528–34.
Ishitobi M, Komoike Y, Motomura K, Koyama H, Inaji H. Early response to neo-adjuvant chemotherapy in carcinoma of the breast predicts both successful breast-conserving surgery and decreased risk of ipsilateral breast tumor recurrence. Breast J. 2010;16:9–13.
Toi M, Nakamura S, Kuroi K, Iwata H, Ohno S, Masuda N, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110:531–9.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jimbo, K., Kinoshita, T., Asaga, S. et al. Oncological safety of breast-conserving surgery after primary systemic chemotherapy in cT3–4 breast cancer patients. Surg Today 45, 1255–1262 (2015). https://doi.org/10.1007/s00595-014-1052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-014-1052-8