Abstract
Purpose
Our previous experimental study of perforated peritonitis in rats proved that peritoneal lavage with strong acid electrolyzed water (SAEW) has no adverse effects, reduces the bacteria count in the ascitic fluid more effectively than saline, and increases the survival rate significantly. Thus, we conducted a randomized controlled study, applying SAEW in the treatment of perforated appendicitis in children.
Methods
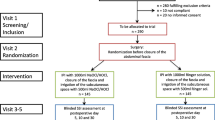
Forty-four patients, aged 3–14 years, were randomly divided into two groups: Group S (n = 20), in which the peritoneal cavity was lavaged with 100 ml/kg saline and the wound was washed out with 200 ml saline; and Group E (n = 24), in which the peritoneal cavity was lavaged with 100 ml/kg SAEW and the wound was washed out with 200 ml SAEW.
Results
No adverse effect of SAEW was observed in Group E. There was no difference in the bacterial evanescence ratio of ascitic fluid after lavage between Groups S and E (11.1 and 15.8 %, respectively). A residual abscess developed in one patient from each group (5.0 and 4.2 %, respectively). The incidence of surgical site infection (SSI) was significantly lower in Group E than in Group S (0 and 20 %, respectively; P < 0.05). There was no difference in the duration of pyrexia, positive C-reactive protein, leukocytosis, or hospital stay between the groups.
Conclusion
Peritoneal lavage and wound washing with SAEW have no adverse effects and are effective for preventing SSI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical site infection (SSI) and residual abscess in the peritoneal cavity remain the important causes of morbidity after perforated peritonitis, resulting in prolonged hospital stay and higher costs [1–3], while compromising the patient’s quality of life. To reduce the incidence of SSI and intraperitoneal abscess, we introduced the use of strong acid electrolyzed water (SAEW) to pediatric cases of perforated peritonitis [4], after confirming its safety and effectiveness in an experimental study [5].
SAEW is generated by electrolysis of a weak salt solution, or 0.2 % sodium chloride (NaCl) and tap water. Our recent experimental study of perforated peritonitis in rats demonstrated that peritoneal lavage with SAEW had no adverse effects, it reduced the bacteria count in the ascitic fluid more effectively than saline, and its use resulted in a significantly increased survival rate [5]. Thus, to confirm its efficacy and safety for peritoneal lavage to reduce the risk of of SSI and residual abscess, we conducted this randomized controlled study of children with perforated appendicitis.
Materials and methods
The subjects of this study were 44 children who underwent appendectomy for perforated appendicitis with extensive or panperitonitis, between 2008 and 2012, at our affiliated hospitals. Patients were allocated randomly to one of two treatment groups: Group S (saline group; n = 20; 16 boys and 4 girls, ranging in age from 4 to 11 years) or Group E (SAEW group; n = 24; aged 3–14 years, 12 boys and 12 girls, ranging in age from 3 to 14 years). Informed consent was obtained in writing from the parents of all patients in the study. Thirty-four patients were excluded from the study because some had received antibiotics before the operation and some had required antibiotics for massive abscess formation to be resected primarily with appendectomy. There was no significant difference in the WBC count (15.8 ± 4.1 × 103 vs. 15.2 ± 6.1 × 103) and CRP value (11.5 ± 7.1 vs. 9.7 ± 5.3 mg/dl) between Groups S and E. The abdominal wall was disinfected with povidone iodine, and laparotomy was performed via a pararectal incision, saving the muscle layers, followed by appendectomy, carried out in the same manner in both groups. After appendectomy, the peritoneal cavity was lavaged with 100 ml/kg saline or SAEW, in Groups S and E, respectively. The peritoneum and fascia were closed with continuous and interrupted monofilament absorbable sutures, respectively. After closure of the fascial layer, the wound was washed out with 200 ml saline or SAEW in Groups S and E, respectively, and the skin was sutured. A drain was not placed in the peritoneal cavity or subcutaneously. Cefmetazole, 100 mg/kg/day, was given initially to both groups, which was replaced by the most sensitive antibiotics after identification of causative pathogens. If the WBC and CRP showed a steady decline, antibiotics were discontinued on postoperative day (POD 5), and if not, they were continued for another 2 days. Laboratory studies were done routinely before surgery and on PODs 1, 3, and 7. The incidences of postoperative intraperitoneal abscess formation, wound infection, duration of pyrexia (defined as a body temperature of 38 °C or higher), duration of a serum level of C-reactive protein higher than 1 mg/dl, leukocytosis (defined as a WBC count of more than 10,000/cm3), and length of hospital stay were compared between the groups. SSI was defined as an infection at the operation site, occurring up to 30 days after surgery [3], with confirmed causative pathogen(s) identical to those of the appendicitis.
SAEW was generated by electrolysis of tap water containing 0.2 % NaCl with a Model OXILYZER OXM-01A (Koken, Tokyo, Japan). It had the following physicochemical properties: pH 2.5–2.7, oxidative reduction potential 1,000–1,1000 mV, with available chlorine (hypochlorous acid) concentration 40 ppm or 1.2 mM.
This study was approved by the Ethical Committee of Osaka Medical Center and Research Institute for Maternal and Child Health (# 224). Statistical analysis was performed by the Chi square test, with significance defined as p < 0.05.
Results
No changes in vital signs were observed during peritoneal lavage with SAEW and no adhesive intestinal obstruction developed in any of the Group E patients. The dominant pathogenic bacteria detected were almost exclusively anaerobic or facultative anaerobic bacteria, such as Escherichia coli and Bacteroides fragilis. There was no difference between Groups S and E in the exponential reduction in the bacterial count after peritoneal lavage (3.8 ± 1.7 vs. 3.8 ± 2.3, respectively), or in the bacterial evanescence ratio of ascitic fluid after lavage (11.1 vs. 15.8 %, respectively). A residual intraperitoneal abscess developed in one patient from each group (5.0 vs. 4.2 %, respectively). The residual abscess in the patient from Group E developed on POD 12 and the pathogenic bacteria was solely E. coli, whereas that in the patient from Group S developed on POD 3 and the pathogenic bacteria included two species of peptostreptococcus as dominant pathogens; namely, B. fragilis, Y-hemolytic Streptococcus, and E. coli. These abscesses were treated with drainage and resolved by PODs 22 and 24, respectively. SSI occurred as abscess formation at the incision sites between PODs 4 and 7, in four patients from Group S and none from Group E. Therefore, the incidence of SSI was significantly lower in Group E than in Group S, at 0 % vs. 20 %, respectively (P < 0.05). Multiple bacterial species were cultured from the SSIs; however, the dominant pathogenic bacteria were exclusively anaerobic or facultative anaerobic, as E. coli in two cases and B. fragilis and Y-hemolytic Streptococcus in one each (Table 1). All of the SSIs were treated with SAEW lavage twice a day and resolved by POD 20. There were no differences between Groups S and E in the duration of pyrexia, at 0.8 ± 1.3 vs. 0.8 ± 0.9 days; positive C-reactive protein, at 5.7 ± 3.3 vs. 5.0 ± 4.2 days; leukocytosis, at 1.8 ± 3.7 vs. 1.8 ± 3.7 days; or hospital stay, at 9.4 ± 4.7 vs. 8.7 ± 4.0 days, respectively.
Discussion
Our previous experimental study revealed that peritoneal lavage with SAEW reduced the bacteria count in ascitic fluid more effectively than saline, resulting in a significantly increased survival rate compared with that of the group lavaged with saline [5]. Moreover, in our previous pilot study, no intraperitoneal abscess developed in patients whose peritoneal cavity was washed with SAEW, although the case number was small and a significant difference was obtained [4]. Therefore, in the present randomized study, we expected that peritoneal lavage with SAEW would achieve a higher bacterial evanescence ratio of ascitic fluid than achieved with saline, and that there would be a significant difference in the incidence of residual intraperitoneal abscess formation between the two groups. The fact that there was no difference in the effect of peritoneal lavage with SAEW vs. that with saline does not mean that SAEW is not more effective than saline, but rather suggests that lavage with more than 100 ml/kg saline is as effective as that with SAEW. Regarding residual abscess formation, intravenous antibiotics are considered to move into the peritoneal cavity and effectively prevent abscess formation. However, wound or subcutaneous lavage with SAEW effectively inhibited the development of SSI. This result can be explained by the low circulation of subcutaneous tissue, or insufficient exposure to intravenous antibiotics, thereby attributing the complete inhibition of SSI to effective disinfection with SAEW. Thus, we conclude that wound lavage with SAEW inhibits SSI development after appendectomy with perforated peritonitis.
The mechanism of the strong bactericidal activity of SAEW was previously explained by the physiological and chemical properties of SAEW; namely, in an aqueous environment with low pH (below 3) and high oxidative reduction potential (above 900 mV), no viable microorganisms were detected [6, 7]. However, Nakagawara and colleagues [8] demonstrated that hypochlorous acid (HOCl) plays a critical role in bactericidal activity and its concentration is quantitatively correlated with bactericidal activity. The strong microbicidal activity inhibits the growth of not only a wide spectrum of bacteria, including methicillin-resistant Staphylococcus [9, 10], but also of viruses, including human immunodeficiency virus and hepatitis B virus [11–13]. Moreover, it deactivates endotoxin directly [14]. One of the most important properties of SAEW is the fact that HOCl, the essential component of the microbicidal activity of SAEW, is generated by immune cells such as neutrophils; thus, HOCl is a major inorganic microbicidal component of innate immunity. By reason of innate immunity, HOCl has extremely low cytotoxicity and is subsequently minimally invasive to tissue [11]; therefore, it can used to safely irrigate body cavities such as the mediastinum [15, 16], peritoneum [4, 17], and skin ulcers [18, 19]. In addition to these biological properties, SAEW is extremely ecological because its breakdown only produces saline and traces of chloride gas. Finally, it is economical because its source is simply unsterilized tap water and salt.
References
Nichols RL. Preventing surgical site infections: a surgeon’s perspective. Emerg Infect Dis. 2001;7:220–4.
Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43:322–30.
Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008;70(S2):3–10.
Kubota A, Hoki M, Yonekura T, Nose N, Hirooka S, Kosumi T, et al. Effectiveness of acidic oxidative potential water in peritoneal lavage for perforated appendicitis. Asian J Surg. 1999;22:282–3.
Kubota A, Nose K, Yonekura T, Kosumi T, Yamauchi K, Oyanagi H. Effect of electrolyzed strong acid water on peritoneal irrigation of experimental perforated peritonitis. Surg Today. 2009;39:514–7.
Baas Beckking LG, Moor D. Limits of the natural environment in terms of pH and oxidation-reduction potentials. J Geol. 1960;68:243–84.
Hotta K. Acid electrolyzed saline solution: its function and medical application. J Jpn Soc Intensive Care Med. 2000;7:97–105.
Nakagawara S, Goto T, Nara M, Ozawa Y, Hotta K, Arata Y. Spectroscopic characterization and the pH dependence of bactericidal activity of the aqueous chlorine solution. Anal Sci. 1998;14:691–8.
Hotta K, Kawaguchi K, Saitoh F, Saito N, Suzuki K, Ochi K, et al. Antimicrobial activity of electrolyzed NaCl solutions: effect on the growth of Streptomyces spp. Actinomycetologica. 1994;l8:51–6.
Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, et al. Hypochlorous acid as a potential wound care agent. part I. stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:65–79.
Abe S, Miya Y, Okuda R. Inactivation of hepatitis B virus by high oxidation potential water (in Japanese with English abstract). Jpn J Conserv Dent. 1994;37:1616–23.
Morita C, Sano K, Morimatsu S, Kiura H, Goto K, Kohno T, et al. Disinfection potential of electrolyzed solutions containing sodium chloride at low concentrations. J Virol Methods. 2000;85:163–74.
Tagawa M, Yamaguchi T, Yokosuka O, Matsutani S, Maeda T, Saisho H. Inactivation of a hepadnavirus by electrolyzed acid water. J Antimicrob Chemother. 2000;46:363–8.
Tanaka N, Fujisawa T, Daimon T, Fujiwara S, Yamamoto M, Abe T. The cleaning and disinfecting of hemodialysis equipment using electrolyzed strong acid aqueous solution. Artif Organs. 1999;23:303–9.
Hayashi H, Kumon K, Yahagi N, Haruna M, Watranabe Y, Matsui J, et al. Successful treatment of mediastinitis after cardiovascular surgery using electrolyzed strong acid aqueous solution. Artif Organs. 1997;21:39–42.
Ohuchi S, Kawazoe K, Ishihara K, Izumoto H, Eishi K. Management with closed irrigation for post-sternotomy mediastinitis. Jpn J Thorac Cardiovasc Surg. 2003;51:511–4.
Takemura M, Iwamoto K, Goshi S. Intraoperative irrigation with super oxidation water for perforated appendicitis (in Japanese). Jpn J Surg. 1999;61:1303–5.
Sekiya S, Ohmori K, Harii K. Treatment of infectious skin defects or ulcers with electrolyzed strong acid aqueous solution. Artif Organs. 1997;21:32–8.
Selkon JB, Cherry GW, Wilson JM, Hughes MA. Evaluation of hypochlorous acid washes in the treatment of chronic venous leg ulcers. J Wound Care. 2006;15:33–7.
Acknowledgments
We thank the Functional Water Foundation (Tokyo, Japan) for providing the scientific information on strong acid electrolyzed water.
Conflict of interest
Akio Kubota and his co-authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubota, A., Goda, T., Tsuru, T. et al. Efficacy and safety of strong acid electrolyzed water for peritoneal lavage to prevent surgical site infection in patients with perforated appendicitis. Surg Today 45, 876–879 (2015). https://doi.org/10.1007/s00595-014-1050-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-014-1050-x