Abstract
Purpose
We reviewed a series of patients who underwent hepatic resection at our institution, to investigate the risk factors for postoperative complications after hepatic resection of liver tumors and for procurement of living donor liver transplantation (LDLT) grafts.
Methods
Between April 2004 and August 2007, we performed 304 hepatic resections for liver tumors or to procure grafts for LDLT. Preoperative volumetric analysis was done using 3-dimensional computed tomography (3D-CT) prior to major hepatic resection. We compared the clinicopathological factors between patients with and without postoperative complications.
Results
There was no operative mortality. According to the 3D-CT volumetry, the mean error ratio between the actual and the estimated remnant liver volume was 13.4%. Postoperative complications developed in 96 (31.6%) patients. According to logistic regression analysis, histological liver cirrhosis and intraoperative blood loss >850 mL were significant risk factors of postoperative complications after hepatic resection.
Conclusions
Meticulous preoperative evaluation based on volumetric analysis, together with sophisticated surgical techniques, achieved zero mortality and minimized intraoperative blood loss, which was classified as one of the most significant predictors of postoperative complications after major hepatic resection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic resection is often performed for hepatocellular carcinoma (HCC) in patients with liver cirrhosis. The mortality and morbidity rates after hepatic resection have declined in recent years because of improved patient selection, surgical techniques, and perioperative care [1–4]. Many major centers have reported good perioperative results after hepatic resection for HCC, with operative mortality rates typically <2% in the high-volume centers in Japan [5, 6], and 5–8% in other countries [7, 8]. The mortality associated with adult living donor liver transplantation (LDLT) is presumed to be 0.5–1.0% [9]; however, the incidence of postoperative complications remains high in comparison with other types of general surgery.
In the 1980s, hepatic resection in the presence of cirrhosis was associated with high mortality, in the range of 10% at our institution [10, 11]. Since then, efforts have been made to reduce both mortality and morbidity: since 1994, nafamostat mesilate has been used perioperatively for hepatic resections, to stabilize the coagulant and fibrinolytic systems; since 1997, an intraoperative bile leakage test has been carried out routinely [12] and a selective hepatic vein-clamping method has been used in all hepatic resections; since 2000, steroid therapy has been given preoperatively to reduce surgical stress [13]. Surgical candidates, especially for right hepatectomy, were selected carefully based on a preoperative volumetric analysis [14] and donor selections were done using volumetric analysis based on 3-dimensional computed tomography (3D-CT) [15]. Based on these technical refinements and postoperative managements, we have performed about one hundred hepatic resections per year and achieved a hospital mortality rate of 0% in the recent year. In this study, we reviewed a series of patients who underwent hepatic resection at our institution, and investigated the risk factors for postoperative complications after hepatic resection for liver tumor and in donors of LDLT.
Methods
Patients
Between April 2004 and August 2007, 304 liver resections were performed at the Department of Surgery and Science, Kyushu University Hospital, without simultaneous procedures such as biliary reconstruction, colorectal resection, or stoma closure. Table 1 lists the indications for hepatic resection. Forty-three patients underwent intraoperative ablation therapy in addition to liver resection, 66 patients underwent right hepatectomy, and 120 patients were donors for LDLT. There were 208 men and 96 women (mean age 53.1 ± 18.5 years, range 19–85 years).
Before surgery, cardiac, pulmonary, and renal functions were evaluated by electrocardiogram, chest X-ray, and blood tests to exclude major disease, which could greatly increase the risk of hepatic resection. Hepatic function was also evaluated by the Child–Pugh classification [16] and the indocyanine green retention rate at 15 min (ICGR15). The definition of diabetes mellitus was a fasting serum glucose level above 126 mg/dL, abnormal results for a 75 g oral glucose tolerant test, or the need for insulin or an oral antihyperglycemic drug to control glucose levels. Preoperative multidetector helical computed tomography (MDCT) images were created with 2-mm thick slices represented on computed tomography (CT) machines. Enhancement was achieved by an intravenous bolus of a contrast nonionic medium (Iopamion, Schering, Erlangen, Germany) at a rate of 5 mL/s. Three-dimensional reconstruction of the liver, remnant liver, and graft was obtained from the MDCT data with Zio M900 (Zio Software Inc., Tokyo, Japan). The indications for hepatic resection and the operative procedure performed for each patient with liver disease were decided according to the preoperative ICGR15, as described previously [3]. Our criteria for hepatic resection were that ascites was not detected, or that it was controlled with diuretics, and a serum total bilirubin level of <2.0 mg/mL. The extent of resection was based on the ICGR15. Patients with an ICGR15 ≥30% were selected for limited resection. Two-thirds of the nontumorous liver parenchyma could be removed if the ICGR15 was ≤10%, whereas less than one-third could be resected if it was 10–19%. Patients with an ICGR15 of 20–29% received single segmentectomy or less. Donor evaluation and selection for LDLT have been described previously [15, 17]. Briefly, the left lobe (LL) is preferred for the graft and is generally used. The right lobe (RL) is chosen if the estimated LL with the caudate lobe volume of the donor is <35% of the standard liver volume of the recipient. If the remnant liver volume is <35% of the total liver volume, the person will be excluded as a donor candidate.
Surgical procedures
Intraoperative ultrasonography was performed to mark the plane of transection. In major liver resection, extraparenchymatous control of the hepatic artery and the portal and hepatic veins was attempted. Parenchymal transection was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA system, Valleylab Inc., Boulder, CO, USA) and the monopolar dissecting sealer (TissueLink, TissueLink Medical Inc.). Inflow vascular control was performed with an intermittent hemi- or total Glisson’s sheath occlusion (Pringle maneuver) and, if required, with a selective hepatic vein-clamping method. Inflow occlusion was applied intermittently with 15 min of occlusion alternating with 5 min of reperfusion. A bile leakage test was performed using an indocyanine green injection via a cannula placed inside the common bile duct through the cystic duct after transection. Two closed-suction drainage tubes were usually placed at the Winslow’s foramen, near the raw surface of the liver.
Postoperative management
Antibiotics were continued for 24 h postoperatively. Oral intake was started on postoperative day (POD) 1 and the drainage tubes were usually removed on POD 3 if no abnormality was observed. All patients underwent routine CT on POD 7 to assess vessel and parenchymal integrities. Operative mortality was defined as intraoperative death, death within 30 days of surgery, or in-hospital death.
Surveillance for complications was carried out prospectively by physicians. The total bilirubin level of the drainage fluid was measured postoperatively, and bile leakage was diagnosed when the level exceeded 5.0 mg/dL and continued for more than 7 days [18]. Ultrasonography-guided paracentesis or insertion of a second drainage tube was performed in patients with an intra-abdominal collection accompanied by fever or a raised white blood cell count. A sample of the intra-abdominal collection was sent for bacteriological culture. Pleural paracentesis was performed to relieve symptoms if the patient had a fever or dyspnea and severe pleural effusion. Local wound complications included wound infection, delayed stitch-out, or keloid formation. Postoperative intra-abdominal bleeding was considered when bleeding from the drainage tubes exceeded 100 mL/h. In this study, postoperative complications were classified as Clavien’s grade II or more [19]. Postoperative liver failure after hepatic resection was diagnosed according to the Belghiti 50–50 criteria (serum bilirubin >50 μmol/L (2.9 mg/dL) and prothrombin ratio <50%) on POD 5 [20].
Statistical analysis
Categorical variables were compared using the Chi-square test and Fisher’s exact test. Continuous variables are presented as the median (range) and compared using the Mann–Whitney U test. Logistic regression analysis was performed to identify the independent variable for postoperative complications. Differences were considered significant at p < 0.05. All statistical analyses were performed using the StatView 5.0 (Abacus Concepts, Berkeley, CA, USA).
Results
Ninety-six of the 304 (31.6%) patients suffered postoperative complications, as detailed in Table 2. The most common was local wound complications, followed by bile leakage. There was no mortality during surgery, within 30 days of surgery, or during the same hospital admission. Only one patient died of rapid growth of recurrent HCC on POD 64, after re-admission to our hospital.
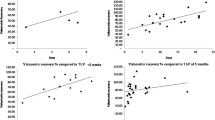
We prospectively investigated the relationship between the actual and the estimated remnant liver volume, using 3D-CT volumetry, in 66 patients who underwent right hepatectomy. The mean actual remnant liver volume and the mean estimated remnant liver volume using 3D-CT volumetry were 497 (range 219–909) and 443 cm3 (range 266–892), respectively. The mean error ratio was 13.4% (range 0.2–59.8; Fig. 1). The mean error ratio was 13.2% (range 0.2–32.7) in the donor group (n = 48) and 13.7% (range 1.9–59.8) in the liver disease group (n = 18). The error ratio of these two groups was not divided significantly. The mean actual and the mean estimated remnant liver volumes using 3D-CT volumetry for 18 patients with liver disease who underwent right hepatectomy were 348 (range 176–605) and 328 mL/m2 (range 232–594), respectively. Five of the 18 patients had an estimated remnant liver volume of <250 mL/m2, using 3D-CT volumetry, but the actual remnant liver volume in three of these patients was greater than 250 mL/m2. Only 1 of 13 patients with an estimated remnant liver volume greater than 250 mL/m2 on 3D-CT volumetry had an actual remnant liver volume of <250 mL/m2. None of these six patients, whose estimated remnant liver volume using 3D-CT volumetry or the actual remnant liver volume was <250 mL/m2, had diabetes mellitus.
Relationship between the actual and the estimated remnant liver volumes based on 3D-CT volumetry. The relationship was linear: y = 49.165 + 1.011 ×x (R = 0.855, p < 0.0001). The mean error ratio was 13.4 ± 9.8%, calculated as follows: error ratio = |E − A|/A × 100 (%). 3D-CT 3-dimensional computed tomography, A actual remnant liver volume (cm3), E estimated graft volume (cm3)
The clinicopathological data for patients with and without complications are compared in Table 3. Those with complications were significantly older (p = 0.0047), a greater proportion had positivity for the hepatitis C virus antibody (HCV-Ab) (p = 0.0380), a greater proportion had diabetes mellitus (p = 0.0192), a lower proportion were donors for LDLT (p = 0.0078), the operation time was ≥490 min (p = 0.0473), intraoperative blood loss was >850 mL (p = 0.0014), and a greater proportion had histological liver cirrhosis (p = 0.0006). The median postoperative stay in hospital was significantly longer in patients with complications.
Logistic regression analysis of factors including age, positivity for HCV-Ab, diabetes mellitus, donor for LDLT, intraoperative blood loss, operation time, and histological liver cirrhosis revealed that histological liver cirrhosis [odds ratio 2.118 (95% confidence intervals (CI) 1.078–4.164); p = 0.0295] and intraoperative blood loss >850 mL [odds ratio 2.167 (95% CI 1.166–4.027); p = 0.0145] were independent predictors of a significantly higher rate of postoperative complications after hepatic resection (Table 4).
In the liver tumor group (n = 184), histological liver cirrhosis [odds ratio 2.096 (95% CI 1.044–4.206); p = 0.0373] and intraoperative blood loss >850 mL [odds ratio 2.735 (95% CI 1.267–5.902); p = 0.0256] were independent predictors of a significantly higher rate of postoperative complications after hepatic resection, based on logistic regression analysis. In the LDLT donor group (n = 120), no variables were identified as predictive of postoperative complications.
Discussion
The surgical techniques for liver resection have improved greatly in recent years, in parallel with decreased postoperative mortality rates. Many large studies have documented acceptable perioperative results, with operative mortality rates typically lower than 2% in the high-volume centers of Japan [5, 6] and 5–8% in other countries [7, 8]. However, there are few series of hepatic resection with no mortality. Midorikawa et al. [18] reported zero mortality in 277 hepatic resections, without postoperative hepatic insufficiency or hospital death, while Imamura et al. [21] reported no operative mortality among 1056 hepatic resections, including donors for LDLT, since 1994. In the present study, we achieved zero mortality among 304 patients who underwent hepatic resections. Moreover, none of our patients suffered liver failure or severe infection, which are common causes of postoperative hospital death. Liver failure is one of the most serious complications of liver resection, and patients with liver cirrhosis are at especially high risk because functional liver tissue is resected from an organ with marginal function. Among 80 patients who underwent major hepatic resections of no less than bisegmentectomy for HCC between 1990 and 1996 at our hospital, 7 died of postoperative liver failure within 6 months. All of the patients who died had undergone right hepatectomy, with a remnant liver volume of <250 mL/m2. According to another study, the only significant risk factor for liver failure in patients with a remnant liver volume of <250 mL/m2 was diabetes mellitus [14]. In this study, 18 of 66 patients who underwent right hepatectomy of the liver had liver disease and 5 of these 18 patients had an estimated remnant liver volume of <250 mL/m2, based on 3D-CT volumetry, but none of these 5 had diabetes mellitus. Thus, postoperative liver failure might occur if the estimated remnant liver volume after right hepatectomy of the liver, using 3D-CT volumetry, is <250 mL/m2 in patients with diabetes mellitus. We think that the strict preoperative patient selection using our criteria, including preoperative ICGR15 and volumetric analysis, preoperative steroid administration to reduce surgical stress, and the perioperative administration of nafamostat mesilate to stabilize the coagulant and fibrinolytic systems, helped to prevent liver failure in patients undergoing hepatic resections.
Preoperative liver volumetry based on 3D-CT has improved outcomes significantly in comparison with that based on 2D-CT [22]. In fact, the estimated remnant liver volume using 3D-CT volumetry was reported to be associated with an error ratio of 5–25% in comparison with the actual remnant liver volume [23, 24]. In our study, the mean error ratio was 13.4%, which is consistent with other reports. Thus, we need to develop methods to decrease the error ratio [25].
Our study clearly demonstrated that histological liver cirrhosis and intraoperative blood loss >850 mL were significant risk factors. Many major centers have published data on mortality and morbidity after hepatic resection for HCC, which usually develops from chronic hepatitis or liver cirrhosis [6–8]. In general, cirrhosis usually causes more problems, including ascites, pleural effusion, septic complications, and liver failure during hepatic resection [26]. Liu et al. [27] reported that underlying liver cirrhosis and intraoperative blood loss >1500 mL were independent and significant factors associated with postoperative morbidity. On the other hand, Chok et al. [28] reported that histological liver cirrhosis did not exist significantly more often in patients with than in those without postoperative complications after hepatic resection for HCC. In the present study, histological liver cirrhosis was an independent risk factor of postoperative complications after hepatic resection for liver tumors and to procure tissue for LDLT from donors with a healthy normal liver. This is compatible with the fact that patients with liver cirrhosis have significantly higher rates of postoperative complications after hepatic resection than those without liver cirrhosis. Recently, transient elastography (FibroScan; Echosens, Paris, France) has become available for the assessment of liver fibrosis as a rapid and noninvasive method of measuring liver stiffness from outside the body [29]. This application might be useful to assess liver cirrhosis preoperatively in patients with chronic liver disease. Intraoperative blood loss is also a well-established risk factor of postoperative complication after hepatic resection [3, 27, 28]. The cutoff intraoperative blood loss volume has been reported as 1500 mL [27]–2000 mL [30]. In the present study, an intraoperative blood loss of >850 mL was found to be a significant risk factor after hepatic resection. The Pringle maneuver and a selective hepatic vein-clamping method help control bleeding in hepatic resection [3]. Moreover, we have recently begun using the monopolar dissecting sealer. These devices and techniques probably contributed to decreasing the mean estimated intraoperative blood loss (687 mL; range 33–4800). Therefore, the cutoff value of the intraoperative blood loss scored lower than reported previously. A randomized controlled trial to verify the validity of using the monopolar dissecting sealer during hepatic resection is warranted.
The incidence of postoperative complications after hepatic resection is reported to range from 20 to 55.5% [1, 8, 28]. In the present study, the overall complication rate was 31.6%, with local wound complications being the most common (20.7%). Patients with local wound complications had significantly greater intraoperative blood loss and a greater proportion of these patients had histological liver cirrhosis (data not shown). Intraoperative blood loss reduces the concentration of antibiotics and is thought to predispose to surgical site infection [31]. Underlying cirrhosis and hypoalbuminemia also inhibit normal wound healing [32]. This concurs with our finding that significantly more patients with histological liver cirrhosis suffered local wound complications.
In conclusion, with strict preoperative clinical evaluation based on volumetric analysis, sophisticated surgical techniques, and careful postoperative management, major hepatic resection can be a safe procedure without a significant risk of mortality. According to our findings, patients with histological liver cirrhosis and intraoperative blood loss >850 mL are at risk of developing postoperative complications.
References
Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–8.
Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46.
Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg. 2007;204(4):580–7.
Hasegawa K, Kokudo N. Surgical treatment of hepatocellular carcinoma. Surg Today. 2009;39:833–43.
Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71–6.
Makuuchi M. Remodeling the surgical approach to hepatocellular carcinoma. Hepatogastroenterology. 2002;49:36–40.
Belghiti J, Regimbeau JM, Durand F, Kianmanesh AR, Dondero F, Terris B, et al. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41–6.
Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41.
Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038 (comment).
Kanematsu T, Takenaka K, Matsumata T, Furuta T, Sugimachi K, Inokuchi K. Limited hepatic resection effective for selected cirrhotic patients with primary liver cancer. Ann Surg. 1984;199:51–6.
Matsumata T, Kanematsu T, Shirabe K, Sonoda T, Furuta T, Sugimachi K. Decreased morbidity and mortality rates in surgical patients with hepatocellular carcinoma. Br J Surg. 1990;77:677–80.
Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45–50.
Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, et al. Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg. 2001;136:328–33.
Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188(3):304–9.
Yonemura Y, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Gion T, et al. Validity of preoperative volumetric analysis of congestion volume in living donor liver transplantation using three-dimensional computed tomography. Liver Transplant. 2005;11:1556–62.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9.
Taketomi A, Kayashima H, Soejima Y, Yoshizumi T, Uchiyama H, Ikegami T, et al. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation. 2009;87:445–50.
Midorikawa Y, Kubota K, Takayama T, Toyoda H, Ijichi M, Torzilli G, et al. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126:484–91.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complication: five-year experience. Ann Surg. 2009;250:187–96.
Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the “fifty-fifty” criteria as an early accurate predictor of death after liver resection in intensive unit patients. Ann Surg. 2009;249:124–8.
Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206.
Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75:1561–4.
Schroeder T, Malago M, Debatin JF, Goyen M, Nadalin S, Ruehm S. All-in-one imaging protocols for the evaluation of potential living liver donors: comparison of magnetic resonance imaging and multidetector computed tomography. Liver Transplant. 2005;11:776–87.
Salvalaggio PR, Baker TB, Koffron AJ, Fryer JP, Clark L, Superina RA, et al. Liver graft volume estimation in 100 living donors: measure twice, cut once. Transplantation. 2005;80:1181–5.
Kayashima H, Taketomi A, Yonemura Y, Ijichi H, Harada N, Yoshizumi T, et al. Accuracy of an age-adjusted formula in assessing the graft volume in living donor liver transplantation. Liver Transplant. 2008;14:1366–71.
Fan ST. Problems of hepatectomy in cirrhosis. Hepatogastroenterology. 1998;45(Suppl 3):1288–90.
Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239(2):194–201.
Chok KS, Ng KK, Poon RT, Yuen WK, Poon RT, Lo CM, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96(1):81–7.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705.
Kobayashi S, Gotohda N, Nakagohri T, Takahashi S, Konishi M, Kinoshita T. Risk factors of surgical site infection after hepatectomy for liver cancers. World J Surg. 2009;33(2):312–7.
Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg. 1996;131(11):1165–71.
Silversein JH. Physiologic changes associated with aging. In: O’Leary JP, editor. The physiologic basis of surgery. 2nd ed. Philadelphia: Lippincott Wikkiams & Wilkins; 2002. p. 705–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itoh, S., Shirabe, K., Taketomi, A. et al. Zero mortality in more than 300 hepatic resections: validity of preoperative volumetric analysis. Surg Today 42, 435–440 (2012). https://doi.org/10.1007/s00595-011-0108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-011-0108-2