Abstract
Introduction
Emerging evidence suggests that HbA1c variability, in addition to HbA1c itself, can be used as a predictor for mortality. The present study aims to examine the predictive power of mean HbA1c and HbA1c variability measures for diabetic complications as well as mortality.
Methods
The retrospective observational study analyzed diabetic patients who were prescribed insulin at outpatient clinics of the Prince of Wales Hospital and Shatin Hospital, Hong Kong, from 1 January to 31 December, 2009. Standard deviation (SD), root mean square (RMS), and coefficient of variation were used as measures of HbA1c variability. The primary outcomes were all-cause and cardiovascular mortality. Secondary outcomes were diabetes-related complications.
Results
The study cohort consists of 3424 patients, including 3137 patients with at least three HbA1c measurements. The low mean HbA1c subgroup had significantly shorter time-to-death for all-cause mortality (P < 0.001) but not cardiovascular mortality (P = 0.920). The high Hba1c subgroup showed shorter time-to-death for all-cause (P < 0.001) and cardiovascular mortality (P < 0.001). Mean Hba1c and Hba1c variability predicted all-cause as well as cardiovascular-specific mortality. In terms of secondary outcomes, mean HbA1c and HbA1c variability significantly predicted diabetic ketoacidosis/hyperosmolar hyperglycemic state/diabetic coma, neurological, ophthalmological, and renal complications. A significant association between dichotomized HbA1c variability and hypoglycemia frequency was found (P < 0.0001).
Conclusion
High HbA1c variability is associated with increased risk of all-cause and cardiovascular mortality, as well as diabetic complications. The association between hypoglycemic frequency, HbA1c variability, and mortality suggests that intermittent hypoglycemia resulting in poorer outcomes in diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus is an increasingly prevalent metabolic disease with a significant global disease burden. Currently, it affects more than 400 million individuals across the globe, with the number of affected patients projected to increase by more than 50% by 2045, and an age-dependent increase in prevalence [1]. Although normalization of blood glucose remains to be the treatment goal for diabetic patients, the extent of glycemic control remains controversial. Previous large-scale clinical trials, such as the United Kingdom Prospective Diabetes Study (UKPDS) and Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), have reported a significant reduction in mortality and cardiovascular complications by tighter glycemic control excluded patients with major comorbidities [2,3,4]. Besides, the 2008 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was ended prematurely due to significantly higher mortality reported in the intensive glycemic control group [5]. There is increasing evidence for increased mortality risk for patients in both extremes of HbA1c, which drives for less stringent glycemic control for the elderly population [6,7,8]. However, the lower limits of glycemic control have yet to be clearly outlined in the current guidelines [9]. Additionally, research has shifted to exploring other parameters that can facilitate more individualized disease-monitoring.
Emerging evidence suggests that HbA1c variability, in addition to HbA1c itself, can be used as a predictor for complications and mortality. Although the underlying mechanism remains unclear, increased HbA1c variability has been associated with diabetic complications in various organ systems, in addition to all-cause and cardiovascular mortality [10,11,12,13]. Different theories have been proposed to explain the association, including that a wide variance in HbA1c may reflect higher complexity in the disease course, suboptimal management, and poorer baseline vascular conditions [14, 15]. Others investigators have proposed the involvement of intermittent hypoglycemia, where the resulting increased oxidative stress and sympathoadrenal activation induce additional stress on end organs under chronic inflammation [16,17,18]. However, there is a lack of evidence from large scale clinical studies to support the hypothesis.
The present study aims to examine the predictive power of both HbA1c value and variability toward the prognosis of diabetic patients. Furthermore, to test the hypothesis that intermittent hypoglycemia underlies the predictive value of HbA1c variability toward prognosis of diabetic patients, the inter-relationship between hypoglycemia, HbA1c variability, and mortality will be evaluated.
Methods
Study population
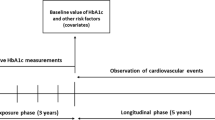
This single-center retrospective observational study was approved by The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. The present study consists of diabetic patients prescribed insulin at outpatient clinics of the Prince of Wales Hospital and Shatin Hospital from January 1, 2009, to December 31, 2009. The patients were identified from the Clinical Data Analysis and Reporting System (CDARS), a territory-wide database that centralizes patient information from individual local hospitals to establish comprehensive medical data, including clinical characteristics, disease diagnosis, laboratory results, and drug treatment details. The system has been previously used by both our team and other teams in Hong Kong [19, 20]. Patients with three or more HbA1c measurements were included in the analysis for HbA1c variability.
Patient data
Clinical and biochemical data were extracted for the present study. Data on the primary outcomes, all-cause and cardiovascular mortality, between January 1, 2009, and May 1, 2019, were obtained. Data on secondary outcomes between January 1, 2009, and December 31, 2013, were extracted, including (1) neurological, ophthalmological and renal diabetic complications, (2) microalbuminuria and macroalbuminuria, (3) peripheral vascular disease, (4) stroke and transient ischemic attack, (5) atrial fibrillation, (6) sudden cardiac death, (7) diabetic ketoacidosis or hyperosmotic hyperglycemic state (DKA/HHS) or coma.
Microalbuminuria was defined as fulfilling any of the following: (1) urine albumin/creatinine ratio between 3 mg/mmol and 30, (2) 24-h total urine albumin between 30 mg/d and 300, (3) spot urine albumin between 30 mg/L and 300. Macroalbuminuria is defined as (1) urine albumin/creatinine ratio > 30 mg/mmol, (2) 24-h total urine albumin > 300 mg/d, (3) spot urine albumin > 300 mg/L. Proteinuria was defined as either 24-h total urine protein > 3.5 g/d, or albumin/creatinine ratio > 30 mg/mmol. Sudden cardiac death was defined as the occurrence of ventricular tachyarrhythmia or non-specific cardiac arrest. Patients with established events before recruitment for a given outcome were excluded. For other outcomes, if there were no events, then these patients were included. Cardiovascular mortality was recorded using the International Classification of Diseases Tenth Edition (ICD-10) coding, while the remaining outcomes were documented in CDARS under ICD-9 codes.
Furthermore, baseline clinical details include: (1) age, (2) sex, (3) specific comorbidities (chronic renal disease [CKD], chronic obstructive pulmonary disease [COPD], chronic liver disease [CLD], heart failure [HF], ischemic heart disease [IHD], hypertension, myocardial infarction [MI], stroke). To capture the episodic occurrence of diseases, such as MI, or the initiation of chronic conditions, such as hypertension, data on patient diagnosis from January 1, 1999, to December 31, 2008, were extracted. The patient’s age is defined as age on January 1, 2009. Additionally, the dosing regimen of antidiabetic and cardiovascular medications prescribed was extracted. The mean daily dose, which is the product between the daily dosing frequency and dosage, is reported for each drug class. The classes of anti-diabetic agents include: (1) insulin, (2) sulphonylurea, (3) biguanide, (4) alpha-glucosidase inhibitor, (5) thiazolidinedione, (6) meglitinide, (7) dipeptidyl peptidase-4 inhibitor, (8) glucagon-like peptide receptor-1 agonist. The cardiovascular medications include: (1) angiotensinogen-converting-enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), (2) beta-adrenergic receptor blocker, (3) calcium channel blocker (CCB), (4) diuretics.
Baseline biochemical data, defined as urinalysis or blood test results measured from January 1, 2008, to December 31, 2008, were extracted. Urinalysis results include: (1) albumin/creatinine ratio, (2) creatinine clearance, (3) spot protein, albumin, and glucose, (4) 24-h total protein, and albumin. Data from renal function test, liver function test, and other baseline blood tests include: (1) serum creatinine, (2) serum sodium, potassium, urate and urea, (3) serum albumin, (4) serum total protein, (5) serum total bilirubin, (6) serum alanine aminotransferase (ALT), (7) alkaline phosphatase (ALP), (8) fasting and random blood glucose, (9) total, high-density lipoprotein (HDL), direct and calculated low-density lipoprotein (LDL) cholesterol, (10) triglyceride. The following results were obtained from complete blood count: (1) hemoglobin, (2) mean corpuscular hemoglobin (MCH), (3) mean corpuscular hemoglobin concentration (MCHC), (4) mean corpuscular volume (MCV), (5) hematocrit, (6) basophil count, (7) eosinophil count, (8) lymphocyte count, (9) monocyte count, (10) neutrophil count, (11) platelet count, (12) red blood cell count, (13) white blood cell count. HbA1c from January 1, 2004, to December 31, 2008, was extracted to establish the baseline HbA1c and HbA1c variability. Random and fasting blood glucose were also extracted to obtain the episodes of hypoglycemia, defined by blood glucose < 3.9 mg/mmol. Results from the latest test that took place in 2008 were used as the baseline if multiple tests were performed during the year.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. HbA1c temporal variability was examined through the following approaches: (1) mean, (2) standard deviation (SD), (3) root mean square (RMS), (4) coefficient of variation (CV). RMS is calculated by first squaring all HbA1c values, then square root the mean of the squares. The coefficient of variation was obtained by dividing the HbA1c standard deviation by the mean HbA1c, expressed as a percentage. Hba1c variability score (HVS) was defined as the number of HbA1c measurements > 0.5% of the previous reading divided by the total number of HbA1c measurements, expressed as a percentage.
Logistic regression was used to identify significant predictors of the different outcomes. Cox regression was applied to evaluate the predictive value of HbA1c variability for time-to-death in all-cause mortality and cardiovascular mortality. Time-to-death was defined by the number of days from January 1, 2009, to the date of death of the patient, or until May 1, 2020. 95% confidence interval (CI) was presented in the analyses, with odds ratio (OR) and hazard ratio (HR) for logistic and Cox regression, respectively.
Several methods were adopted to further outline the relationship between blood glucose value, HbA1c variability, and mortality. The relationship between mean HbA1c and time-to-death for both all-cause and cardiovascular mortality was modeled using the generalized additive model, with 95% CI displayed. Cut-off values of HbA1c mean and SD for the prediction of all-cause and cardiovascular mortality were derived by the maximization of sensitivity and specificity, using the Liu method, and the area under the reactive operator characteristic (ROC) curve was calculated. SD was used to represent HbA1c variability since it is the only parameter unrelated to the value of HbA1c amongst the four parameters for variability.
The cohort was then dichotomized into “high” and “low” HbA1c value and variability based on the HbA1c mean and SD, respectively. Kaplan–Meier curves were plotted for the dichotomized cohorts against the time-to-death for all-cause mortality, with the significance of intergroup differences assessed by the log-rank test. To elucidate the underlying connections between intermittent hypoglycemia, HbA1c variability, and mortality, Poisson regression was used to assess the relationship between hypoglycemia frequency and dichotomized HbA1c variability, while both logistic and Cox regression were used to test the predictive ability of hypoglycemia frequency toward both all-cause and cardiovascular mortality. Statistical significance is defined as P value < 0.05. All statistical analyses were performed using R Studio.
Results
Clinical and biochemical characteristics
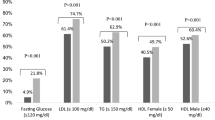
The present cohort consists of 3424 patients (median age = 63, interquartile range of age = 20 years, male = 50.2%). A flowchart of inclusion and exclusion for arriving at this cohort is shown in Supplementary Fig. 1. The baseline biochemical parameters of the cohort are presented in Table 1. Within the present cohort, the most common comorbidity is hypertension (24.6%), followed by IHD (15%), stroke (11%), HF (10%), CKD (7%), CLD (5%), MI (4%) and COPD (3%). In terms of drug prescriptions, the mean daily insulin dose was 20.8 ± 13.0 units. Other classes of antidiabetic agents prescribed include: (1) biguanide (n = 1300, mean daily dose = 1546 ± 742 mg); (2) sulphonylurea (n = 1300, mean daily dose = 131 ± 122 mg); (3) thiazolidinedione (n = 268, mean daily dose = 7.17 ± 8.93 mg); (4) alpha-glucosidase inhibitor (n = 89, mean daily dose = 185 ± 80.0 mg); (5) glucagon-like peptide-1 receptor agonist (n = 8, mean daily dose = 11.8 ± 4.05 mg); (6) dipeptidyl peptidase-4 inhibitor (n = 7, mean daily dose = 97.8 ± 14.9 mg). The following cardiovascular medications were prescribed: (1) ACEI/ARB (n = 2328, mean daily dose = 20.6 ± 38.9 mg); (2) CCB (n = 1579, mean daily dose = 58.2 ± 49.0 mg); (3) beta-adrenergic receptor blockers (n = 1425, mean daily dose = 79.3 ± 64.3 mg); (4) diuretics (n = 955, mean daily dose = 66.3 ± 82.2 mg).
HbA1c variability and baseline
Within the study cohort, 3137 patients had at least three HbA1c measurements, and the average number of HbA1c measurements per patient was 11.9 ± 4.8. The average frequency of hypoglycemia was 0.6 ± 1.3 episodes. Throughout the study period (1, 2009–31 December 2019), there were 1491 cases of all-cause mortality, within which 308 were attributed to cardiovascular causes. Overall, the mean baseline HbA1c is 8.1 ± 1.8% (interquartile range = 2.0%), with HbA1c variability represented by (1) patient-specific mean (8.0 ± 1.2%); (2) SD (1.1 ± 0.71%); (3) RMS (8.1 ± 1. 2%); (4) CV (13.6 ± 7.6%). The logistic and Cox regression analysis for outcome prediction is presented in Table 2 and Table 3, respectively.
For the prediction of the primary outcomes—all-cause and cardiovascular mortality, both baseline HbA1c and HbA1c variability were found to be significant predictors. Interestingly, under logistic regression, baseline (OR = 0.89, 95% CI = [0.85, 0.93], P < 0.001), mean (OR = 0.90, 95% CI = [0.85, 0.95], P < 0.001), and RMS (OR = 0.92, 95% CI = [0.87, 0.97], P < 0.001) of HbA1c were found to be negative predictors of all-cause mortality, while SD (OR = 1.34, 95% CI = [1.21, 1.49], P < 0.001), CV (OR = 1.03, 95% CI = [1.02, 1.04], P < 0.001), and HVS (OR = 1.007, 95% CI = [1.004, 1.009], P < 0.001) were found to be significant positive predictors. Similar findings were found under Cox regression, with baseline (HR = 0.92, 95% CI = [0.89, 0.95], P < 0.001), mean (HR = 0.91, 95% CI = [0.87, 0.95], P < 0.001), and RMS (HR = 0.93, 95% CI = [0.89, 0.97], P < 0.001) as negative predictors, SD (HR = 1.21, 95% CI = [1.14, 1.28], P < 0.001), CV (HR = 1.02, 95% CI = [1.02, 1.03], P < 0.001) and HVS (HR = 1.01, 95% CI = [1.01, 1.01], P < 0.001) as positive predictors for time-to-death.
Similar patterns were observed in cardiovascular mortality. While baseline HbA1c is a negative predictor for both cardiovascular-specific mortality (OR = 0.89, 95% CI = [0.82, 0.96], P = 0.005) and time-to-death (HR = 0.90, 95% CI = [0.84, 0.98], P = 0.009), SD (mortality: OR = 1.42, 95% CI = [1.23, 1.66], P < 0.001; time-to-death: HR = 1.34, 95% CI = [1.21, 1.48], P < 0.001), CV (mortality: OR = 1.03, 95% CI = [1.02, 1.05], P < 0.001; time-to-death: HR = 1.03, 95% CI = [1.02, 1.04], P < 0.001) and HVS (mortality: HR = 1.02, 95% CI = [1.01, 1.03], P < 0.001; time-to-death: HR = 1.02 95% CI: [1.01, 1.03], P < 0.001) are positive predictors.
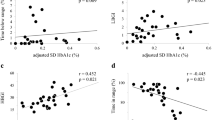
The individual effects of HbA1c and blood glucose values, in addition to HbA1c variability, on all-cause and cardiovascular mortality were further examined. The longer time-to-death at the extremes of mean HbA1c in the generalized additive model for all-cause mortality is presented in Fig. 1. The cut-off values for dichotomization of HbA1c value and variability were 7.3% (area under the curve [AUC] = 0.540) and 0.86 (AUC = 0.574) in all-cause mortality prediction, and 6.8% (AUC = 0.493) and 0.88 (AUC = 0.590) for cardiovascular mortality prediction. After dichotomization, the low mean HbA1c subgroup showed a significantly shorter time-till-death for all-cause mortality, (P < 0.001), but not cardiovascular mortality (P = 0.920). By contrast, the time-till-death was significantly shorter for the high HbA1c variability subgroup for both all-cause (P < 0.001), and cardiovascular mortality (P < 0.001).
The Kaplan–Meier plots for the dichotomized HbA1c value and variability in the prediction of all-cause and cardiovascular mortality are presented in Figs. 2a-d, and the corresponding Cox plots are presented in Supplementary Figs. 2a–d. A significant association was found between dichotomized HbA1c variability and hypoglycemia frequency (P < 0.0001). Hypoglycemia frequency was found to be a positive predictor for both mortality (OR = 1.11, 95% CI = [1.05, 1.17], P < 0.001) and time-till death of all-cause (HR = 1.08, 95% CI = [1.04, 1.11], P < 0.001), in addition to cardiovascular-specific time-till-death (HR = 1.07, 95% CI = [1.00, 1.15], P = 0.044).
In terms of prediction of secondary outcomes, both baseline HbA1c and HbA1c variability are significant positive predictors for the following:
-
1.
DKA/HHS/coma (baseline: OR = 1.16, 95% CI = [1.04, 1.28], P = 0.006; mean: OR = 1.32, 95% CI = [1.14, 1.52], P < 0.001; SD: OR = 1.27, 95% CI = [1.02, 1.53], P = 0.018; RMS: OR = 1.29, 95% CI = [1.13, 1.47], P < 0.001; HVS: OR = 1.011, 95% CI = [1.003, 1.018], P = 0.004);
-
2.
Neurological diabetic complications (baseline: OR = 1.07, 95% CI = [1.00, 1.14], P = 0.033; mean: OR = 1.16, 95% CI = [1.05, 1.28], P = 0.003; SD: OR = 1.23, 95% CI = [1.07, 1.42], P = 0.004; RMS: OR = 1.15, 95% CI = [1.05, 1.27], P = 0.002; CV: OR = 1.02, 95% CI = [1.01, 1.03], P = 0.006; HVS: OR = 1.01, 95% CI = [1.00, 1.01], P = 0.001);
-
3.
Ophthalmological diabetic complications (baseline: OR = 1.10, 95% CI = [1.05, 1.15], P < 0.001; mean: OR = 1.32, 95% CI = [1.23, 1.42], P < 0.001; RMS: OR = 1.28, 95% CI = [1.19, 1.37], P < 0.001);
-
4.
Only mean HbA1c and RMS were predictive for microalbuminuria (mean: OR = 1.18, 95% CI = [1.08, 1.29], P < 0.001; RMS: OR = 1.16, 95% CI = [1.07, 1.27], P < 0.001) and proteinuria (mean: OR = 1.25, 95% CI = [1.14, 1.37], P < 0.001; RMS: OR = 1.23, 95% CI = [1.13, 1.34], P < 0.001);
-
5.
Renal diabetic complications were predicted by mean (OR = 1.25, 95% CI = [1.03, 1.19], P = 0.006), SD (OR = 1.14, 95% CI = [1.02, 1.28], P = 0.025), RMS (OR = 1.10, 95% CI = [1.03, 1.18], P = 0.007) and HVS (OR = 1.005, 95% CI = [1.002, 1.008], P = 0.002);
-
6.
Baseline HbA1c (OR = 1.11, 95% CI = [0.999, 1.23], P = 0.041) and HVS (OR = 1.014, 95% CI = [1.006, 1.023], P = 0.001).
Discussion
The major findings of the present study are that: (1) both HbA1c value and variability can predict mortality and complications in diabetics; (2) low HbA1c was associated with higher all-cause mortality; (3) the frequency of hypoglycemia episodes was associated with HbA1c variability; (4) hypoglycemia frequency was predictive of both all-cause and cardiovascular mortality in diabetic patients.
In the past, a positive linear relationship is perceived between HbA1c value and all-cause mortality [21, 22]. However, emerging evidence from large cohort studies suggests that there is instead an increased all-cause mortality risk at both low and high HbA1c levels [7, 11, 23, 24]. In the present study, a similar U-shaped association was demonstrated for the time-till-death of both all-cause and cardiovascular mortality against HbA1c value under the generalized additive model. The increased all-cause and cardiovascular mortality risk amongst patients with low HbA1c value are demonstrated by the poorer survival of the low mean HbA1c subgroup, and the baseline HbA1c value showing hazard ratios less than unity for both mortality and time-till-death. Although the underlying mechanism between low HbA1c and increased mortality remains unclear, low HbA1c has been associated with chronic inflammation and liver function derangement [25]. Furthermore, a similar U-shaped relationship has been reported for blood glucose against endothelial dysfunction and frailty [26,27,28]. These findings, therefore, suggest that more relaxed glycemic control for the older patient with greater frailty may improve patient outcome.
The present study provides further evidence of the predictive value of HbA1c variability. Similar to previous studies, high HbA1c variability is associated with increased risk in both all-cause and cardiovascular mortality, in addition to vascular, neurological, ophthalmological, and renal complications [10, 12, 14, 29, 30]. Overall, HbA1c variability demonstrated greater predictive value for directly diabetes-caused complications in different organ systems, but its predictive value was limited for other associated conditions, such as stroke. The statistical insignificance in the prediction of cardiovascular events, contrary to existing studies, may be attributed to the single-center nature of the study cohort, which limited the incidence of associated conditions.
Although the mechanism between HbA1c variability and diabetes-induced complications remains unclear, possible explanations involving intermittent hypoglycemia were raised. Some investigators suggested that intermittent hypoglycemia induces the production of reactive oxygen species, and the increased oxidative stress results in endothelial dysfunction, which ultimately leads to cardiovascular complications and death [31,32,33,34]. Another potential theory is that hypoglycemic episodes stimulate sympathetic activation, which stresses the cardiovascular system and affect the end-organ blood supply [16]. Therefore, to elucidate the biological connections between HbA1c variability and diabetic progression, the present study examined the inter-relationship between hypoglycemia, HbA1c variability, and diabetic outcomes. Dichotomized HbA1c variability was found to be a positive predictor of hypoglycemic episodes, while hypoglycemia itself was a positive predictor for mortality. Therefore, it can be inferred that the predictive value of HbA1c variability is at least partially contributed by the effects of intermittent hypoglycemia.
Despite the emerging evidence for the predictive value of HbA1c variability, its clinical application remains limited by the absence of a standardized quantification method. Existing studies employed methods such as counting the frequency of significant successive differences, percentage deviation from the expected trajectory, SD, CV, and HVS [11, 12, 29, 35]. With different variability parameters, the present study demonstrates that the calculation of variability may be affected by the HbA1c value, and the number of measurements taken. The resulting difference in the inherent sensitivity of the parameters results in the difference in their predictive power. Therefore, when HbA1c variability is used for prediction, different parameters have to be adopted to reduce the effect of confounding factors.
Limitations
Several limitations should be noted for the present study. Firstly, the size of the study cohort is limited by its single-centered nature. The resulting limitation in the incidence of diabetes-related disease events may affect the predictive power. Furthermore, like other observational studies, it is limited by potential under-coding of comorbidities, missing data, and coding errors. Additionally, the duration of diabetes, diabetic progression, and treatments prescribed were not accounted for, which can affect the interpretation of HbA1c value and variability. Given prior studies from different country of origin reporting the association between Hba1c variability and adverse outcomes, we speculate that our findings can be generalizable to other populations. However, the external validity of the study should be confirmed in non-Chinese cohorts.
Conclusion
In conclusion, the present study demonstrated the association between high HbA1c variability and increased risk for both all-cause and cardiovascular mortality, in addition to diabetic complications across different organ systems. The association between hypoglycemic frequency, HbA1c variability, and mortality support the hypothesis that intermittent hypoglycemia contributes to poor outcomes in diabetic patients. Further research on larger cohorts is required to provide further evidence for the predictive value of HbA1c variability on the prognosis of diabetic patients and to shed light on its associations with hypoglycemia.
References
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
King P, Peacock I, Donnelly R (1999) The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 48(5):643–648. https://doi.org/10.1046/j.1365-2125.1999.00092.x
Heller SR, Group AC (2009) A summary of the advance trial. Diabetes Care 32(Suppl 2):S357–S361. https://doi.org/10.2337/dc09-S339
Huang ES, Davis AM (2015) Glycemic control in older adults with diabetes mellitus. JAMA 314(14):1509–1510. https://doi.org/10.1001/jama.2015.8345
Buse JB, Bigger JT et al (2007) Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 99(12A):21i–33i. https://doi.org/10.1016/j.amjcard.2007.03.003
Currie CJ, Peters JR, Tynan A et al (2010) Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375(9713):481–489. https://doi.org/10.1016/S0140-6736(09)61969-3
Anyanwagu U, Mamza J, Donnelly R, Idris I (2019) Relationship between HbA1c and all-cause mortality in older patients with insulin-treated type 2 diabetes: results of a large UK cohort study. Age Ageing 48(2):235–240. https://doi.org/10.1093/ageing/afy178
Arnold LW, Wang Z (2014) The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev Diabet Stud 11(2):138–152. https://doi.org/10.1900/RDS.2014.11.138
American Diabetes A (2020) 12. Older adults standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S152–S162. https://doi.org/10.2337/dc20-S012
Gorst C, Kwok CS, Aslam S et al (2015) Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 38(12):2354–2369. https://doi.org/10.2337/dc15-1188
Forbes A, Murrells T, Mulnier H, Sinclair AJ (2018) Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 6(6):476–486. https://doi.org/10.1016/S2213-8587(18)30048-2
Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER (2020) Visit-to-visit HbA1c variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care 43(2):426–432. https://doi.org/10.2337/dc19-0823
Su JB, Zhao LH, Zhang XL et al (2018) HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol 17(1):47. https://doi.org/10.1186/s12933-018-0693-0
Luk AO, Ma RC, Lau ES et al (2013) Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong diabetes registry. Diabetes Metab Res Rev 29(5):384–390. https://doi.org/10.1002/dmrr.2404
Waden J, Forsblom C, Thorn LM et al (2009) A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 58(11):2649–2655. https://doi.org/10.2337/db09-0693
Wright RJ, Frier BM (2008) Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 24(5):353–363. https://doi.org/10.1002/dmrr.865
Kahal H, Halama A, Aburima A et al (2020) Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci Rep 10(1):4750. https://doi.org/10.1038/s41598-020-61531-z
Wang J, Alexanian A, Ying R et al (2012) Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32(3):712–720. https://doi.org/10.1161/ATVBAHA.111.227389
Li CK, Xu Z, Ho J et al (2020) Association of NPAC score with survival after acute myocardial infarction. Atherosclerosis 301:30–36. https://doi.org/10.1016/j.atherosclerosis.2020.03.004
Ju C, Lai RWC, Li KHC et al (2019) Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/kez576
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352(9131):854–865
Andersson C, van Gaal L, Caterson ID et al (2012) Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes. Diabetologia 55(9):2348–2355. https://doi.org/10.1007/s00125-012-2584-3
Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358(24):2545–2559. https://doi.org/10.1056/NEJMoa0802743
Li W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G (2016) HbA1c and all-cause mortality risk among patients with type 2 diabetes. Int J Cardiol 202:490–496. https://doi.org/10.1016/j.ijcard.2015.09.070
Carson AP, Fox CS, McGuire DK et al (2010) Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes 3(6):661–667. https://doi.org/10.1161/CIRCOUTCOMES.110.957936
Crane PK, Walker R, Hubbard RA et al (2013) Glucose levels and risk of dementia. N Engl J Med 369(6):540–548. https://doi.org/10.1056/NEJMoa1215740
Rogers SC, Zhang X, Azhar G, Luo S, Wei JY (2013) Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J Gerontol A Biol Sci Med Sci 68(12):1469–1481. https://doi.org/10.1093/gerona/glt033
Zaslavsky O, Walker RL, Crane PK, Gray SL, Larson EB (2016) Glucose levels and risk of frailty. J Gerontol A Biol Sci Med Sci 71(9):1223–1229. https://doi.org/10.1093/gerona/glw024
Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ (2018) Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol 17(1):96. https://doi.org/10.1186/s12933-018-0739-3
Cheng D, Fei Y, Liu Y et al (2014) HbA1C variability and the risk of renal status progression in diabetes mellitus: a meta-analysis. PLoS ONE 9(12):e115509. https://doi.org/10.1371/journal.pone.0115509
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295(14):1681–1687. https://doi.org/10.1001/jama.295.14.1681
Costantino S, Paneni F, Battista R et al (2017) Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 66(9):2472–2482. https://doi.org/10.2337/db17-0294
Tse G, Lai ET, Tse V, Yeo JM (2016) Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J Diabetes Res 2016:2848759. https://doi.org/10.1155/2016/2848759
Tse G, Yan BP, Chan YW, Tian XY, Huang Y (2016) Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol 7:313. https://doi.org/10.3389/fphys.2016.00313
Skriver MV, Sandbaek A, Kristensen JK, Stovring H (2015) Relationship of HbA1c variability, absolute changes in HbA1c, and all-cause mortality in type 2 diabetes: a Danish population-based prospective observational study. BMJ Open Diabetes Res Care 3(1):e000060. https://doi.org/10.1136/bmjdrc-2014-000060
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethical approval
This single-center retrospective observational study was approved by The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee.
Informed consent
Informed consent was waived by the Ethics Committee due to the retrospective, observational nature of the study.
Additional information
Managed By Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S., Liu, T., Zhou, J. et al. Predictions of diabetes complications and mortality using hba1c variability: a 10-year observational cohort study. Acta Diabetol 58, 171–180 (2021). https://doi.org/10.1007/s00592-020-01605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01605-6