Abstract
Aims
To compare the long-term functional and anatomical outcomes of cataract surgery with combined versus 1-month deferred intravitreal dexamethasone implant (DEX) in eyes with pre-existing diabetic macular edema (DME).
Methods
Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were retrospectively evaluated in both groups before treatments, then 1, 4, 12 and 24 months after DEX.
Results
Forty eyes were analyzed, 20 in each group. BCVA disclosed comparable trends, increasing from similar starting values (p = 0.9913) to akin scores 1 month after DEX (p = 0.4229). After 4 months, it similarly reduced without significant variations within each group throughout the whole observation period. CRT was similar at the time of surgery (p = 0.6134) and was reduced by DEX injection in both samples, with a superior beneficial effect in the combined group after 1 month (p = 0.0010). At 4 months, CRT further elevated and remained overall stable in the long term without differences. By 12 months, 19 (95%) eyes received further injections: 1 (5%) fluocinolone, 3 (15%) received other DEX and fluocinolone, 13 (65%) ≥ 1 DEX only and 2 (10%) anti-VEGFs. During the second year, 6 additional eyes (from the 13 receiving DEX) switched to fluocinolone, reaching a total of 10 (50%). Similar results were observed in the deferred group.
Conclusions
DEX implant performed at the time of surgery achieved the same long-term functional and anatomical outcomes compared to a 1-month injection deferral in treating eyes with pre-existing DME that should undergo cataract extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given the increasing global prevalence of diabetes together with the longer life expectancy and aging of the population, cataract surgery in diabetic individuals is going to overall be increasingly frequent [1].
In the diabetic eye two different entities, with distinct pathogenesis and natural histories, may contribute to macular edema after surgery: the onset of postcataract macular edema (PCME) and the worsening of pre-existing diabetic macular edema (DME) [2, 3]. Diabetic patients have an increased and independent risk of developing PCME, even after uncomplicated surgeries, higher in the presence of any diabetic retinopathy (DR) [4, 5]. On the other hand, pre-existing DME, even fovea-sparing, represents itself the principal risk factor for edema worsening after uneventful phacoemulsification [2, 6, 7]. These two singularities may unexpectedly combine and precipitate the outcome, making cataract surgery in eyes with DME actually challenging.
Currently, specific guidelines about DME management during cataract surgery have not been published, leaving surgeons to individual experience. The widespread availability and the rich literature about intravitreal anti-vascular endothelial growth factor (VEGF) agents and steroid-releasing systems have globally revolutionized DME management and therapy. However, there is a limited experience of DME pharmacotherapy in combination with cataract surgery [8,9,10,11,12,13,14,15]. Indeed, their advantageous effects in treating pre-existing DME and/or preventing worsening after a major inflammatory stimulus, such as phacoemulsification, have been well documented [16, 17]. Particularly, intravitreal steroid slow-releasing agents, despite the risk of intraocular pressure elevation, are effective in the treatment of DME [18,19,20,21]. Specifically, dexamethasone 700-μg implant (DEX) is a steroid-releasing agent purposely designed and registered for intravitreal use for treating different retinal vascular problems, including DME. The intraocular bioavailability is averaged to last up to 6 months.
Few case series recently reported the protective and beneficial effects of intravitreal DEX implant performed at the time of surgery [22,23,24,25]. This research aims to evaluate the long-term functional and anatomical outcomes of eyes undergoing cataract surgery combined with DEX implant in eyes with chronic persistent DME, compared with a homogeneous sample undergoing the same procedure but with a 1-month deferred steroid injection.
Methods
In this retrospective observational study, we made a structured research of all consecutive patients who underwent combined cataract surgery and intravitreal from January 2010 to October 2017 at our institution. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this study. All the procedures performed involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee of the San Raffaele Scientific Institute and with the 1964 Helsinki Declaration and its later amendments. All patients signed an informed consent form, which was specifically designed and approved by the Institutional Ethics Committee of the IRCCS San Raffaele Hospital for participants of retrospective studies.
Data search
A specific query to the general institutional electronic medical chart (EMC) was made, asking for all subjects who underwent the combination of the ICD-9 procedures 13.41 (phacoemulsification and aspiration of cataract), 13.71 (artificial lens insertion) and 14.75 (injection of vitreous substitute). Output database was cleaned according to the inclusion and exclusion criteria, and the constituted group was named “combined.”
The control sample was gathered by means of the institutional ophthalmological custom-made EMC NIDEK nLIFE and named “deferred” group.
Investigational sample inclusion/exclusion criteria
Clinical information was collected by NIDEK nLIFE EMC and imaging review.
Inclusion criteria were: age ≥ 18 years and diagnosis of pre-existing and recurrent (nontreatment-naïve) central-involving DME, inactive diabetic retinopathy [non-proliferative diabetic retinopathy (NPDR) or inactive laser-treated proliferative diabetic retinopathy (PDR)], availability of clinical and imaging data at least for the first 24-month observational points of the study design. Pre-existing and recurrent DME was defined as a DME that failed to achieve a complete resolution after any treatment. Subjects with this DME were hence approaching cataract surgery in a context where their macula could not be completely and reasonably dried. According to the optical coherence tomography (OCT) classification of DME, recently proposed by Panozzo et al. [26], we selected early and advanced DME.
Exclusion criteria were: intravitreal injection in the previous 5 months before cataract surgery, HbA1c > 9%, any other concurrent retinal diseases (e.g., retinal vein occlusion, retinal dystrophies, age-related macular degeneration, etc.), glaucoma, previous vitrectomy. Eyes with any significant optical media opacity that could interfere with a good-quality imaging acquisition were also excluded.
Control sample inclusion/exclusion criteria
Clinical information was collected by NIDEK nLIFE EMC and imaging review, specifically requesting for a population of subjects with the same diagnosis of pre-existing and recurrent early or advanced DME and: age ± 5 years of the investigational group average age (for statistic matching purposes), cataract surgery procedure, DEX implant done 4–6 weeks after cataract surgery. Similarly, availability of clinical and imaging data at least for the first 24-month observational points of the study design was mandatory. Exclusion criteria were the same of the investigational group.
Study design
In the investigational group, data were collected in the following time points: baseline (the same day of combined phacoemulsification and DEX implant injection, right before the procedure), 1, 4, 12 and 24 months after procedure. The clinical data of the control group were collected in the following time points: the day of surgery (right before the procedure), the day of DEX implant injection (baseline), then according to the same time points of the investigational group.
The following clinical information was acquired:
-
General data (sex, age).
-
Medical history, including glycated hemoglobin (HbA1c) and DR stage.
-
Best-corrected visual acuity (BCVA) that per-protocol was measured with the Early Treatment Diabetic Retinopathy Study charts.
-
Central retinal thickness (CRT) at SD-OCT, measured using the inbuilt Spectralis software (Heidelberg Engineering, Heidelberg, Germany).
Procedures
All surgeries were performed by one surgeon under topical anesthesia and using a standard surgical procedure. Briefly, a continuous curvilinear capsulorhexis was accomplished through a 2.4-mm temporal clear corneal incision. After hydrodissection, phacoemulsification of the nucleus and cortical aspiration were performed. An acrylic hydrophobic single-piece intraocular lens was then placed in the capsular bag.
Before removing the eye speculum, dexamethasone 700-μg implant was administered at the end of cataract extraction. The applicator system provided by the manufacturer was used to insert the implant into the vitreous through a pars plana puncture.
All patients had the same postsurgical medication regimen: topical fluoroquinolone 4 times a day for 1 week, topical dexamethasone 4 times a day for 1 week and then tapered for the following 2 weeks, a topical nonsteroidal anti-inflammatory drug for 3 weeks.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8.0.0 for Mac (GraphPad software, San Diego, California, USA).
The Mann–Whitney test was used to compare the outcome measures between the two groups. The Friedman test was used to compare the outcome measure within the same group throughout the follow-up. The Fisher’s exact test was used to assess differences for noncontinuous variables. In all analyses, p values < 0.05 were considered significant.
Results
Initial characteristics
A total of 43 eyes of 40 patients (23 females and 17 males) undergoing the combination of ICD-9 13.41, 13.71 and 14.75 procedures were extrapolated. Among these, 12 were treated for other diseases (macular edema due to retinal vein occlusion, wet age-related macular degeneration, uveitis). Of the remaining 31 diabetic eyes, 6 were treated with an intravitreal anti-VEGF, 4 were lost to follow-up, and 1 was treated with DEX implant 4 months before surgery.
Twenty eyes fulfilling the inclusion/exclusion criteria were finally analyzed (“combined” group). Surgery was successfully carried out in all the 20 investigational eyes without any intraoperative or postoperative complication. Intravitreal DEX implant was performed at the end of surgery, as a standard intravitreal injection. In these eyes, diabetic macular edema had been previously treated with anti-VEGF in 1 case (5%), with DEX in 7 cases (35%) and with the both in 12 cases (60%).
Twenty control eyes were extrapolated and considered as controls (“deferred” group). These eyes underwent a deferred dexamethasone implant injection 37 ± 3 days after cataract surgery. In these eyes, diabetic macular edema had been previously treated with anti-VEGF in 1 case (5%), with DEX in 9 cases (45%) and with the both in 10 cases (50%).
The initial characteristics (before cataract surgery) of the two samples are reported in Table 1. No differences were noted between the two groups in terms of gender distribution, age, HbA1c level, BCVA and CRT. A similar relative frequency of PDR was also found that had been treated in all cases with peripheral laser photocoagulation and judged by the clinician inactive.
Functional outcomes
Visual acuity significantly changed after treatments within the same group throughout the follow-up, but was found to be similar in each of the time points of the research between the two samples (Table 2).
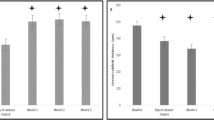
In the combined group, using the post hoc Dunn’s multiple comparison test, BCVA was increased 1 month after concurrent surgery and DEX injection (p < 0.0001) and then decreased at the 4-month control (p = 0.0093). No further significant changes occurred at 12 and 24 months (p nonsignificant for all) (Fig. 1a).
In the deferred group, BCVA raised after surgery (p = 0.0149) and after the DEX performed approximately 1 month later (p = 0.0098), reaching similar values to the combined group. BCVA then decreased at the 4-month visit (p = 0.0005), remaining stable at the 12- and 24-month time-point observations (Fig. 1a).
Anatomical outcomes
Central retinal thickness disclosed slightly different trends between the two groups.
Both combined and deferred groups had similar CRT at the time of surgery (p = 0.6134). Within the deferred group, a significant CRT increase was noted after phacoemulsification (p = 0.0139), which reduced after DEX injection (p = 0.0001). The combined sample, compared to deferred, was found to have an average thinner macula at the time of injection (baseline—Table 3). CRT after combined therapies reached inferior thickness compared to deferred group after 1 month (p < 0.0001) (Table 3, Fig. 1b).
At 4 months both groups disclosed a similar significant CRT increase, without other differences in the subsequent observation time points in both groups. Figure 2 provides illustrative sample cases of combined and deferred anatomical course over 2 years.
Adverse events
No serious adverse events, such as endophthalmitis or retinal detachment, were reported in this study. Ocular hypertension (intraocular pressure > 25 mmHg) was found in 2 eyes of the deferred group (10%) at the 1-month control, and in 1 eye (5%) of the combined group, similarly at the 1-month control. All were successfully treated with topical therapy.
Further treatments after dexamethasone
The outcomes of patient undergoing cataract surgery with combined or deferred DEX implant intravitreal injection were observed over a period of at least 24 months. Throughout this period, eyes were followed in real-world setting and treated as needed according to the clinician’s findings.
After the first DEX implant injection (baseline) and by the 12-month time-point observation, out of the 20 eyes of the investigational group 19 (95%) received further injections. One eye (5%) was treated with fluocinolone acetonide implant, 3 (15%) received 1 dexamethasone and 1 fluocinolone, 13 (65%) underwent DEX injections only (at least 1), and 2 (10%) treated with anti-VEGFs (Fig. 3a).
Throughout the second year from baseline, from the 12-month and by the 24-month observation points, 6 additional eyes (from the 13 previously treated with DEX only) switched to fluocinolone, reaching a total of 10 (50%) fluocinolone-treated eyes. The remaining 7 DEX-treated eyes continued to receive the same drug, with one additional eye, which was not treated during the first year. The 2 anti-VEGF-treated eyes maintained the same therapy. All eyes were hence under further therapy.
Similar results were observed in the deferred group (Fig. 3b), where 5 (25%) (p = 0.18) underwent fluocinolone treatment during the 12-month observation period. By the 24-month observation point 13 eyes (65%) were under fluocinolone treatment (p = 0.5231).
Two eyes under fluocinolone acetonide received further anti-VEGF injections, due to edema relapse.
Discussion
Facing cataract surgery for a diabetic patient is a challenging problem. It has been indeed thoroughly documented that diabetic eyes have an increased and independent risk of developing PCME, even after uncomplicated surgeries, and regardless of the DR stage [2, 6, 7]. The relative risk of PCME is increased in patients with DR, and it proportionately rises with increasing severity of DR [4, 5].
At present dedicated guidelines about DME management whenever cataract surgery is required are not available, leaving surgeons to individual experience. It is understandably desirable, and generally accepted, that it would be better to undergo surgery with a dry macula. But there are challenging cases requiring cataract extraction where DME cannot be eliminated, being considered chronic and persistent. Having pre-existing DME, even fovea-sparing, represents itself the principal risk factor for edema worsening after uneventful phacoemulsification, owing to the combination of the procedural inflammatory load with the chronic macula inflammatory levels.
Combining intravitreal steroid injection at the time of cataract surgery is a strategy to face this specific, but critical, issue. Corticosteroids retain indeed fast pharmacokinetic and efficacious dynamic effects. A widespread diffusion of this combined strategy has been limited yet by administrative issues, such as reimbursement and procedural concerns.
Few case series recently reported the protective and beneficial effects of intravitreal DEX implant performed at the time of surgery [22,23,24,25]. Our research was specifically designed for this purpose, looking for a population undergoing cataract with a pre-existing and recurrent DME treated with surgery combined with DEX 700-μg implant. Our aim was to analyze the functional and anatomical outcomes in the long term, compared with a matched sample undergoing separate deferred procedures.
The combination of the two procedures was found to be safe and effective in treating macular edema after cataract surgery. No procedure-related adverse effects were registered.
The functional trend was similar between the two groups. In the combined sample BCVA significantly increased 1 month after phacoemulsification with DEX injection and then dropped down at the end of the implant pharmacologic activity. Visual acuity showed an overall stability throughout the follow-up, being similar at 1 and 2 years. In the deferred group BCVA expectedly disclosed a two-step augmentation, after surgery first and after DEX injection, achieving similar values at each time point of the observational period. These data suggest that the functional short-term and long-term outcomes might be considered similar while deciding to combine or defer steroid injection to treat DME.
Anatomical outcomes revealed interesting considerations. Despite disclosing similar trends, CRT showed some differences between the two groups. Macular thickness significantly reduced after combined treatments in the 1st month and then raised back at the end of DEX effects at 4 months. Average CRT values remained overall stable in the long term. Deferring the two procedures was found to be effective, but CRT disclosed higher values than the combined groups at the 1st month that is considered to be the most pharmacoactive moment of steroid release. Specifically, macular thickness expectedly increased significantly after surgery and reduced after deferred steroid injection, yet without reaching the anatomical effects of the combination of the two procedures. We hypothesize that the inflammatory load of surgery is not probably merely cumulated with the pre-existing DME, but is exponentially combined resulting in a clinically relevant form of edema that is less responsive to DEX injection. At the end of DEX pharmacoactivity CRT dropped back to similar values and comparably behaved throughout follow-up.
Our results come out in favor of the combination of the two procedures. Despite disclosing similar morphofunctional outcomes in the long term, two principal rationales can support this evidence. First, combining DEX at the time of cataract surgery might contribute to protect the diabetic eye during an acute and strong inflammatory stimulus such as the one of surgery. Despite losing its effect after the pharmacoactive window, DEX might prevent short-term subclinical damages during the first postsurgical months as demonstrated by significant difference in CRT at 1 month post-DEX (combined versus deferred). Second, the procedural combination may reduce the burden of visits and examination, both for patients and for caregivers. One may figure out a scenario where a diabetic patient is approaching surgery and must undergo: pre-op visit, surgery, 1- and 7-day controls, 1-month visit with OCT; then, whether DME should be treated: scheduling injection, injection, control of injection, 1-month visit after injection. This burdensome and potentially delaying process can be simplified with a combined procedure, requiring just pre-op visit with OCT, and following per-protocol visits.
The combination of cataract surgery with a perioperative steroid injection gives rise to doubts about the possible increased risks of endophthalmitis. Despite our reduced sample size, this was never observed in our cases, finding agreement with similar published studies [24, 25]. It might be argued that full asepsis with povidone-iodine and proper eye draping are efficacious in avoiding infective complications. We also experienced a low rate of increased intraocular pressure, which were all successfully resolved with medical therapy.
The long-term analysis also provides remarkable clinical information. After the first DEX injection and by the first year, 95% of the combined groups received further injections, the majority of which (65%) were further DEX injections. Among the remaining, 5% directly switched to fluocinolone acetonide, 15% received at least 1 further DEX and then switched to fluocinolone and 10% were only treated anti-VEGFs. Throughout the second year from baseline, additional eyes (from the ones previously treated with DEX only) switched to fluocinolone, reaching a total 50% fluocinolone-treated eyes. All eyes were hence under further therapy. The deferred group revealed similar nonsignificant differences, with a 30% of eyes by the first year and 65% by the second year under fluocinolone acetonide.
Our results find agreement with the available literature, which supports the advantage of combined dexamethasone with cataract surgery in terms of functional and anatomical outcomes [22,23,24,25]. Our investigation further confirms those data, but strengthens the results by adding information about long-term follow-up and about the comparison with a homogeneous matched group with a deferred steroid injection.
The possible role of nonsteroidal anti-inflammatory drugs in reducing PCME should be also disclosed, as this might have confounded the beneficial effects of steroids. The postsurgical eye drop protocol is, by the way, standard and was homogeneous between the two groups.
Our study has several drawbacks that must be disclosed. First, the reduced number of included eyes statistically and clinically limits the inferences. Though it should be considered that the combination of the two procedures is a relatively infrequent occasion, data collection was restricted. Second, the absence of patient standardization raises the outcome variability. This is unavoidably correct, but it should be considered that facing cataract surgery is intrinsically not standardized, as it is individualized in the patient–doctor relationship. We optimized this aspect carefully selecting eyes where persistent DME was present and not treated in the previous 5 months before surgery. Although BCVA and OCT are comparable between the two groups, we further increased homogeneity excluding cases of severe DME and atrophic maculopathy, associated with a worst response to treatment and visual acuity [26]. Eventually, it might also be said that there is a lack of data in the intermediate observation point (e.g., 6 months or 18 months). This was not possible owing to the retrospective study design, as patients were enrolled from the real world, where specific time-point observation is frequently not available. In addition, it could also be argued that the DEX deferral after cataract might not be considered the most appropriate choice for some patients. It has thought to be considered, as above-mentioned, that our data were retrospectively derived from the real world. DEX timing, either prior or subsequent to cataract surgery, was indeed decided by the clinician and planned according to the center availability. Our research limited to analyze those receiving injection 1 month after surgery.
Despite the limitations, we believe this study offers a real-world scenario of a very specific, but unexpectedly frequent and crucial, aspect of the care process of a diabetic patient. There are different aspects that cannot be ignored, while the ophthalmologists administer cures, especially when the best clinical options meet the best solutions for the quality of life of these patients, last but not least whenever economical burdens are unload.
Combining DEX injection together with cataract surgery during the same procedure is a safe and clinically effective option that should be considered to prevent the inflammatory procedural load and to ameliorate the care process.
References
Panozzo G, Staurenghi G, Dalla Mura G et al (2019) Prevalence of diabetes and diabetic macular edema in patients undergoing senile cataract surgery in Italy: the DIabetes and CATaract study. Eur J Ophthalmol. https://doi.org/10.1177/1120672119830578
Hayashi K, Igarashi C, Hirata A, Hayashi H (2009) Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 23:389–396. https://doi.org/10.1038/sj.eye.6703022
Sarao V, Veritti D, Maurutto E et al (2018) Pharmacotherapeutic management of macular edema in diabetic subjects undergoing cataract surgery. Expert Opin Pharmacother 19:1551–1563
Chu CJ, Johnston RL, Buscombe C et al (2016) Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology 123:316–323. https://doi.org/10.1016/j.ophtha.2015.10.001
Denniston AK, Chakravarthy U, Zhu H et al (2017) The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group, Report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol 101:1673–1678. https://doi.org/10.1136/bjophthalmol-2016-309838
Dowler JG, Sehmi KS, Hykin PG, Hamilton AM (1999) The natural history of macular edema after cataract surgery in diabetes. Ophthalmology 106:663–668. https://doi.org/10.1016/S0161-6420(99)90148-3
Il KS, Hwang DJ, Seo JY, Park IW (2011) Evaluation of changes of macular thickness in diabetic retinopathy after cataract surgery. Korean J Ophthalmol 25:238–242. https://doi.org/10.3341/kjo.2011.25.4.238
Chen C-H, Liu Y-C, Wu P-C (2009) The combination of intravitreal bevacizumab and phacoemulsification surgery in patients with cataract and coexisting diabetic macular edema. J Ocul Pharmacol Ther 25:83–89. https://doi.org/10.1089/jop.2008.0068
Akinci A, Muftuoglu O, Altınsoy A, Ozkılıc E (2011) Phacoemulsification with intravitreal bevacizumab and triamcinolone acetonide injection in diabetic patients with clinically significant macular edema and cataract. Retina 31:755–758. https://doi.org/10.1097/IAE.0b013e3182006da1
Rauen PI, Ribeiro JAS, Almeida FPP et al (2012) Intravitreal injection of ranibizumab during cataract surgery in patients with diabetic macular edema. Retina 32:1799–1803. https://doi.org/10.1097/IAE.0b013e31824bebb8
Lam DSC, Chan CKM, Mohamed S et al (2005) Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular oedema: a 6-month prospective pilot study. Eye (Lond) 19:885–890. https://doi.org/10.1038/sj.eye.6701686
Habib MS, Cannon PS, Steel DHW (2005) The combination of intravitreal triamcinolone and phacoemulsification surgery in patients with diabeticfoveal oedema and cataract. BMC Ophthalmol 5:15. https://doi.org/10.1186/1471-2415-5-15
Nunome T, Sugimoto M, Kondo M, Suto C (2018) Short-term results of intravitreal triamcinolone acetonide combined with cataract surgery for diabetic macular edema in japan: in the era of anti-vascular endothelial growth factor therapy. Ophthalmologica 240:73–80. https://doi.org/10.1159/000487548
Lim LL, Morrison JL, Constantinou M et al (2016) Diabetic macular edema at the time of cataract surgery trial: a prospective, randomized clinical trial of intravitreous bevacizumab versus triamcinolone in patients with diabetic macular oedema at the time of cataract surgery—preliminary 6 month results. Clin Exp Ophthalmol 44:233–242. https://doi.org/10.1111/ceo.12720
Ozgur OR, Ozkurt Y, Kulekci Z, Evciman T (2016) The combination of phacoemulsification surgery and intravitreal triamcinolone injection in patients with cataract and diabetic macular edema. Saudi J Ophthalmol Off J Saudi Ophthalmol Soc 30:33–38. https://doi.org/10.1016/j.sjopt.2015.10.004
Murtha T, Cavallerano J (2007) The management of diabetic eye disease in the setting of cataract surgery. Curr Opin Ophthalmol 18:13–18. https://doi.org/10.1097/ICU.0b013e32801129fc
Fraser-Bell S, Kaines A, Hykin PG (2008) Update on treatments for diabetic macular edema. Curr Opin Ophthalmol 19:185–189
Lattanzio R, Cicinelli MV, Bandello F (2017) Intravitreal steroids in diabetic macular edema. Dev Ophthalmol 60:78–90. https://doi.org/10.1159/000459691
Sacconi R, Giuffrè C, Corbelli E et al (2019) Emerging therapies in the management of macular edema: a review [version 1; peer review: 2 approved]. F1000 Research Ltd, London
Sacconi R, Parodi MB, Casati S et al (2017) Dexamethasone implants in diabetic macular edema patients with high visual acuity. Ophthalmic Res 58:125–130. https://doi.org/10.1159/000477256
Sacconi R, Corbelli E, Carnevali A et al (2018) Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol 102:1684–1690. https://doi.org/10.1136/bjophthalmol-2017-311240
Sze AM, Luk FO, Yip TP et al (2015) Use of intravitreal dexamethasone implant in patients with cataract and macular edema undergoing phacoemulsification. Eur J Ophthalmol 25:168–172. https://doi.org/10.5301/ejo.5000523
Agarwal A, Gupta V, Ram J, Gupta A (2013) Dexamethasone intravitreal implant during phacoemulsification. Ophthalmology 120(211):211.e1–5. https://doi.org/10.1016/j.ophtha.2012.08.002
Furino C, Boscia F, Niro A et al (2017) Combined phacoemulsification and intravitreal dexamethasone implant (Ozurdex®) in diabetic patients with coexisting cataract and diabetic macular edema. J Ophthalmol 2017:4896036. https://doi.org/10.1155/2017/4896036
Panozzo GA, Gusson E, Panozzo G, Dalla Mura G (2017) Dexamethasone intravitreal implant at the time of cataract surgery in eyes with diabetic macular edema. Eur J Ophthalmol 27:433–437. https://doi.org/10.5301/ejo.5000920
Panozzo G, Cicinelli MV, Augustin AJ et al (2020) An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol 30:8–18. https://doi.org/10.1177/1120672119880394
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Corbelli, Dr. Fasce, Dr. Iuliano, Dr. Sacconi and Dr. Lattanzio have nothing to disclose. Dr. Bandello reports personal fees from Alcon (Fort Worth, Texas, USA), personal fees from Alimera Sciences (Alpharetta, Georgia, USA), personal fees from Allergan Inc. (Irvine, California, USA), personal fees from Farmila-Thea (Clermont-Ferrand, France), personal fees from Bayer Schering Pharma (Berlin, Germany), personal fees from Bausch And Lomb (Rochester, New York, USA), personal fees from Genentech (San Francisco, California, USA), personal fees from Hoffmann-La-Roche (Basel, Switzerland), personal fees from Novagali Pharma (Évry, France), personal fees from Novartis (Basel, Switzerland), personal fees from Sanofi-Aventis (Paris, France), personal fees from ThromboGenics (Heverlee, Belgium), personal fees from Zeiss (Dublin, USA), outside the submitted work. Dr. Querques reports personal fees from Alimera Sciences (Alpharetta, Georgia, USA), personal fees from Allergan Inc. (Irvine, California, USA), personal fees from Amgen (Thousand Oaks, USA), personal fees from Heidelberg (Germany), personal fees from KBH (Chengdu, China), personal fees from LEH Pharma (London, UK), personal fees from Lumithera (Poulsbo, USA), personal fees from Novartis (Basel, Switzerland), personal fees from Bayer Schering Pharma (Berlin, Germany), personal fees from Sandoz (Berlin, Germany), personal fees from Sifi (Catania, Italy), personal fees from Soof-Fidia (Albano, Italy), personal fees from Zeiss (Dublin, USA), outside the submitted work.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Corbelli, E., Fasce, F., Iuliano, L. et al. Cataract surgery with combined versus deferred intravitreal dexamethasone implant for diabetic macular edema: long-term outcomes from a real-world setting. Acta Diabetol 57, 1193–1201 (2020). https://doi.org/10.1007/s00592-020-01509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01509-5