Abstract
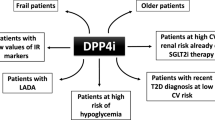
Achieving and maintaining recommended glycemic targets without causing adverse e ffects, including hypoglycemia, is challenging, especially in older patients with type 2 diabetes mellitus (T2DM). The introduction of dipeptidyl peptidase-4 (DPP-4) inhibitors, more than 10 years ago, has provided an alternative to conventional medications for the intensification of glucose-lowering treatment after failure of metformin monotherapy, and therefore, marked an important advance in the management of T2DM. By prolonging the activity of incretin hormones, DPP-4 inhibitors induce insulin release and decrease glucagon secretion in a glucose-dependent manner. This results in a more physiologic glycemic control as compared to that ensured by insulin secretagogues (sulfonylureas and glinides). Overall, DPP-4 inhibitors have a favorable safety profile and can be used without dose adjustments in older adults and in patients with mild renal impairment; they have a neutral effect on body weight and do not cause hypoglycemia by themselves. Safety issues, reported mainly in post-marketing surveillance programs and including cardiovascular outcomes and the risk of acute pancreatitis, are being extensively investigated. The aim of this review is to discuss the impact of DPP-4 inhibitors on the treatment of T2DM, after 10 years of experience, with an emphasis on diabetes care in Italy. We will first describe T2DM treatment in Italy and then provide an overview of the main findings from randomized controlled trials, real-world studies and post-marketing surveillance programs with DPP-4 inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a widespread, chronic and complex disease which is associated with severe complications including chronic kidney disease (CKD) and cardiovascular (CV) disease [1, 2]. T2DM largely affects the elderly population, with a prevalence of approximately 25% among individuals aged ≥ 65 years [3,4,5]. Achieving and maintaining recommended glycemic targets without causing adverse effects, such as hypoglycemia and weight gain, is challenging when using traditional antihyperglycemic medications. This problem is particularly relevant in older patients [4, 6, 7].
The oral glucose-lowering dipeptidyl peptidase-4 (DPP-4) inhibitors, or gliptins, were introduced for the treatment of T2DM 10 years ago [8]. By inhibiting DPP-4, a protease that degrades the incretin hormones glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), gliptins prolong the activity of incretins leading to increased postprandial glucose-dependent insulin secretion and decreased glucagon secretion [9, 10]. The main representatives of this class are vildagliptin, sitagliptin, saxagliptin, linagliptin, and alogliptin [11,12,13,14,15]. DPP-4 inhibitors are indicated in adult patients with T2DM as an adjunct to diet and exercise, as monotherapy when metformin is not tolerated or contraindicated, or in combination with other antihyperglycemic medications, including insulin. DPP-4 inhibitors are characterized by a low risk of hypoglycemia and neutral effects on body weight [10]. Current guidelines recommend DPP-4 inhibitors as add-on to metformin or other glucose-lowering agents in dual or triple therapy [16, 17].
The aim of this review is to discuss the impact of DPP-4 inhibitors on the treatment of T2DM, after 10 years of experience, with an emphasis on diabetes care in Italy. We will first deal with T2DM treatment in Italy and the position of DPP-4 inhibitors in routine diabetes practice. We will then provide an overview of the main findings from randomized controlled trials (RCTs) and real-world studies with DPP-4 inhibitors.
The current status of diabetes care in Italy
According to the Italian National Institute of Statistics (ISTAT), the known cases of diabetes in Italy were > 3 million in 2016, corresponding to a prevalence of > 5.3% of the entire population [18]. Estimates based on the data collected by the Italian ARNO Osservatorio Diabete—a database launched in 2007 to document treatment patterns of diabetes in Italy—have shown a prevalence of 6.3% which may underestimate the true prevalence of the disease, as diabetes patients that are not treated pharmacologically are not recorded in the database [3]. Furthermore, diabetes remains undiagnosed in a substantial proportion of patients. More than 60% of the diabetes patients in the ARNO database were aged more than 65 years and about one-fifth were more than 80 years.
According to the ISTAT, the standardized mortality rate due to diabetes was 28.4 per 100,000 Italian residents in 2014 [18]. Over the past 5 years, an improvement in the management of patients with diabetes has occurred as highlighted by the decrease in the hospitalization rate due to T2DM from 22.4/100,000 in 2010 to 13.7/100,000 in 2015 [18]. This improvement was observed across all Italian Regions [18]. CKD and CV disease are the most frequent complications associated with T2DM, with an estimated prevalence of 37.5% and 23.2%, respectively, among T2DM patients, according to the Italian multicenter RIACE study [19]. A recent study comparing the incidence of major CV events, deaths and drug prescriptions in patients with or without diabetes in a Northern Italian Region found a decrease in the incidence of CV morbidity and mortality between 2002 and 2012 in both groups [20]. These findings were paralleled by an increase, over the same period, in the use of drugs for CV disease and for diabetes.
With regard to the prescription of antihyperglycemic drugs in Italy, recent data from the Medicines Utilization Monitoring Centre (OsMed) of the Italian medicines agency (AIFA) have shown a considerable reduction from 2015 to 2016 in the use of insulins and insulin analogues [21]. The utilization of sulfonylureas in association with metformin or as monotherapy has also decreased from 2015 to 2016, while a 6.8% increase in the use of DPP-4 inhibitors has been reported [21]. According to the ARNO database mentioned above, in 2016 metformin was the most frequently used oral antihyperglycemic (62.2% of 516,073 treated individuals) followed by sulfonylureas (19.2%), repaglinide (8.9%), and metformin plus sulfonylureas (8.2%) [3]. DPP-4 inhibitors were used by 7.0% of patients in combination with metformin and by 5.0% as monotherapy. Among the five currently available gliptins, sitagliptin was the most frequently used in association with metformin or alone. The other newer oral antihyperglycemics, GLP-1 analogues and sodium-glucose cotransporter-2 (SGLT-2) inhibitors, were used by 2.4% and 2.5% of patients recorded in the ARNO database, respectively.
The relatively low use of DPP-4 inhibitors in Italy can be explained, at least in part, by the prescribing limitations issued by the AIFA [22]. According to these limitations, incretin-based therapies are reimbursed only if they are prescribed by a diabetes specialist. Furthermore, the prescription of incretin-based drugs is restricted to cases of therapeutic failure defined by levels of glycated hemoglobin (HbA1c) ≥ 7.5% (≥ 58 mmol/mol) with the maximum tolerated dose of the current glucose-lowering agent [22]. In addition, HbA1c levels must be < 8.5–9.0% (< 69–75 mmol/mol), a limit established based on the fact that the average HbA1c reduction associated with incretin-based therapy is ≤ 1% [22]. Evidence suggests that current prescribing regulations for incretin-based therapies result in limited access to appropriate treatment for patients not referred to diabetes centers.
In contrast, an analysis of electronic health record systems and national registers in Finland showed that DPP4-inhibitors were the most common agent for treatment intensification after metformin failure and that the increased use of DPP4-inhibitors occurred after the introduction of a policy for full reimbursement (in 2010) [23]. Similar to Italy, treatment intensification tended to occur at relatively high HbA1c levels (at approximately 7.5%, 58 mmol/mol), which is higher than recommended international and international guidelines (at 7.0%, 53 mmol/mol) [23].
According to the 2015 data from the ARNO database concerning the diabetes population aged ≥ 65 years, a substantial proportion of elderly patients were treated with insulin secretagogues (43.4%), including sulfonylureas and glinides, which are associated with a higher risk of hypoglycemia compared with other oral antihyperglycemics [24]. These findings are in line with the results from real-life observational studies. For example, the RIACE study showed that sulfonylureas and repaglinide are widely used in clinical practice in elderly patients with impaired renal function and that their use tend to increase with increasing age [25]. According to the ARNO database, only 10.7% of elderly patients were treated with DPP-4 inhibitors alone or in association with metformin, and the proportion of older patients treated with gliptins decreased with increasing age [24]. In Italy, the underprescribing of gliptins to older T2DM patients after failure of metformin monotherapy may also be a consequence of the fact that these patients, who are usually treated by general practitioners (GPs), will more likely be prescribed the add-on of traditional medications, like sulfonylureas, than be referred to a diabetologist for prescription of DPP-4 inhibitors.
T2DM management 10 years after the introduction of DPP-4 inhibitors
The development of DPP-4 inhibitors was made possible by the improvements in our understanding of the pathophysiology of diabetes leading to the design of drugs able to target the mechanisms responsible for hyperglycemia and, therefore, to provide a more physiologic control of blood glucose [9]. The introduction of DPP-4 inhibitors has brought about an important conceptual change in the management of T2DM and caused a shift from an approach focused on lowering glucose levels to strategies targeting the underlying pathophysiological processes [8]. DPP-4 inhibitors have also contributed to the recognition of hypoglycemic adverse events and weight gain as important clinical barriers to optimal glucose-lowering therapy [8].
Specifically targeting some of the various causes of hyperglycemia, newly introduced agents—gliptins as well as GLP-1 receptor agonists and SGLT-2 inhibitors—have substantially enlarged the available therapeutic armamentarium, which now includes as many as 7 classes of non-insulin anti-hyperglycemia drugs. This large armamentarium allows physicians to tailor anti-hyperglycemic treatment to fit the specific needs of each individual patient and to achieve personalized glucose/HbA1c targets with an improved safety profile [7].
Compared with other oral antihyperglycemics, DPP-4 inhibitors induce a less rapid and marked glucose-lowering effect. This more gradual effect on glucose levels is counterbalanced by a more favorable safety profile: gliptins have a neutral effect on body weight and, by stimulating insulin secretion in a glucose-dependent manner, they do not cause hypoglycemia by themselves [26,27,28,29]. Thus, the introduction of DPP-4 inhibitors has no doubt provided a valid and overall safer alternative to insulin secretagogues (sulfonylureas and glinides) for the intensification of glucose-lowering treatment after failure of metformin monotherapy [27]. Furthermore, DPP-4 inhibitors can be used in elderly patients and in the presence of CKD [11,12,13,14,15, 30]. Of note, large real-life observational studies comparing the effectiveness of DPP-4 inhibitors vs sulfonylureas have demonstrated that the addition of a DPP-4 inhibitor to ongoing therapy allows for better glycemic control than the addition of a sulfonylurea, and that HbA1c-lowering is directly proportional to baseline HbA1c levels and indirectly proportional to T2DM duration [31, 32]. Evidence also exists that treatment with gliptins potentiate glucose-dependent insulin secretion in T2DM and, more recently, in latent autoimmune diabetes in adults [33,34,35,36].
Overall, DPP-4 inhibitors are characterized by intermediate glucose-lowering efficacy, low risk of hypoglycemia, neutral effect on body weight, low frequency of side effects, and high costs [17, 37]. Efficacy appears comparable among class members, but very few head-to-head comparisons of gliptins are available. Vildagliptin and sitagliptin were the first to be granted marketing authorization by the European Medicines Agency (2007) followed by saxagliptin (2009), linagliptin (2011), and alogliptin (2013). All five gliptins can be used in elderly patients and in the presence of mild renal impairment, with no need for dose adjustments [11,12,13,14,15]. Table 1 summarizes relevant information concerning the treatment with each member of the gliptin family.
DPP-4 inhibitors have been extensively studied in clinical trials over the past decade and a number of studies are ongoing. Real-world evidence supporting the effectiveness of DPP-4 inhibitors is also available. In the next sections, relevant efficacy, safety, and effectiveness of DPP-4 inhibitors will be discussed.
Efficacy of DPP-4 inhibitors
General diabetes population
Most trials with DPP-4 inhibitors were designed to test the agents as add-on to the standard treatment of T2DM, usually metformin. In these trials, DPP-4 inhibitors were compared with placebo or with other add-on treatments. Several systematic reviews and meta-analyses of trials evaluating DPP-4 inhibitors have been performed [26, 28, 29, 38,39,40,41,42,43,44,45].
In an evaluation of 63 systematic reviews of DPP-4 inhibitors for the treatment of T2DM, all DPP-4 inhibitors were shown to significantly reduce HbA1c levels compared with placebo and had similar ability to metformin in reducing HbA1c levels, but were less effective when compared with GLP-1 receptor agonists and sulfonylureas [44]. Similarly, a Bayesian network meta-analysis of 58 randomized controlled trials (n = 31,356 participants with T2DM), which assessed the efficacy and safety of DPP-4 inhibitors compared with placebo and with each other, showed a decrease in HbA1c for all DPP-4 inhibitors vs placebo, except for alogliptin; vildagliptin 50 mg twice daily showed the highest probability in reduction effect on HbA1c [45]. In addition, all DPP-4 inhibitors, and especially linagliptin 10 mg once daily, significantly reduced fasting plasma glucose when compared with placebo [45].
The efficacy of DPP-4 inhibitors on HbA1c levels appears to fall within a suitable range. A meta-regression analysis of 78 RCTs evaluating the efficacy of gliptins on HbA1c levels and including 20,503 patients with T2DM estimated a mean decrease of HbA1c levels from baseline [mean baseline HbA1c 8.03% (64 mmol/mol)] of − 0.74% [46]. Treatment duration ranged from 12 to 104 weeks. The analysis found that baseline HbA1c and fasting glucose levels influenced the response to gliptins, with greater HbA1c reductions being achieved in patients with higher baseline HbA1c and lower fasting glucose levels.
Overall, the evidence from trials comparing DPP-4 inhibitors with sulfonylureas, the oral antihyperglycemics most commonly used as add-on to metformin in the second-line setting, shows a greater efficacy of sulfonylureas in lowering HbA1c levels during the first 3 months’ of treatment, while DPP-4 inhibitors have demonstrated efficacy in the long-term maintenance of glycemic control. A meta-analysis including 12 RCTs of ≥ 18-week duration, which compared head-to-head DPP-4 inhibitors with sulfonylureas in T2DM patients (n = 10,982, overall), found that sulfonylureas lowered HbA1c significantly more than DPP-4 inhibitors [28]. However, DPP-4 inhibitors had a more favorable safety profile than sulfonylureas, as highlighted by a reduction in body weight, total adverse events and CV events, and a lower risk of hypoglycemia [28]. A meta-analysis of 16 studies with 15,176 patients (8,047 treated with a DPP-4 inhibitor and 7,129 with a sulfonylurea) specifically addressed the efficacy of gliptins vs sulfonylureas, both as add-on therapy to metformin [42]. Sulfonylureas were associated with a significantly greater reduction in HbA1c from baseline to 12 weeks; however, at 52 and 104 weeks, differences between the two treatments were no longer statistically significant. At all-time points, sulfonylureas, but not DPP4-inhibitors, were associated with weight gain. The incidence of hypoglycemia at 12, 52, and 104 weeks was substantially and significantly greater with sulfonylureas (20%, 24%, and 27%, respectively) compared with DPP-4 inhibitors (6%, 3%, and 4%, respectively). Notably, the proportion of patients achieving, at 52 and 104 week, the recommended target of HbA1c < 7% (< 53 mmol/mol), without hypoglycemia, was significantly higher among patients on DPP-4 inhibitors.
Patients with renal impairment
Metformin, the recommended first-line option in T2DM patients not adequately controlled by diet and lifestyle interventions, is contraindicated in patients with moderate–severe renal impairment. Before the introduction of the newer oral antihyperglycemics, these patients had few treatment options. The fact that renal dysfunction is associated with an increased risk of hypoglycemia further complicates the management of these patients [30]. Several studies have shown that DPP-4 inhibitors are effective in lowering HbA1c levels in patients with T2DM and renal impairment, without increasing the risk of hypoglycemia or other adverse events, especially vs active comparators [30, 47,48,49,50,51]. This is reflected in the labels of the five approved gliptins, according to which, these agents are not contraindicated in patients with mild–moderate renal dysfunction (Table 1) [11,12,13,14,15].
Elderly patients
Evidence from more recent studies has, however, shown that DPP-4 inhibitors can also be used successfully in patients aged ≥ 65 years and with long-standing disease. Older T2DM patients are a difficult-to-treat group as they often have multiple comorbidities and are at increased risk of severe hypoglycemia due to different age-related conditions, including progressive renal failure, CV disease, reduced glucagon secretion, and hypoglycemia unawareness [4]. According to national and international guidelines, older adults can use all currently available glucose-lowering agents [4, 16, 17]. When selecting the glucose-lowering agent, great care must be taken to prevent hypoglycemic events, especially in this age group. The cognitive status of the patient, the drug–drug interactions related to polytherapy, and the CV risk must be carefully evaluated, as well, to provide a care plan as individualized as possible.
Studies of DPP-4 inhibitors in older adults have clearly suggested the potential of gliptins for the management of T2DM in this important age group [6, 7, 52,53,54,55,56]. A summary of efficacy outcomes of DPP-4 inhibitors in elderly patients with T2DM is shown in Table 2 [6, 7, 53, 56,57,58,59,60]. Alogliptin was shown to be non-inferior to glipizide with consistent and comparable glycemic control in mildly hyperglycemic elderly T2DM patients [59] and to be effective and well tolerated in elderly T2DM patients (aged ≥ 65 years) with similar HbA1c improvements to younger patients (aged < 65 years) and no increased risk of hypoglycemia, weight gain, or other adverse events [58]. Linagliptin improved glycaemia and had a safety profile similar to placebo in elderly T2DM patients with inadequate glycemic control despite receiving other glucose-lowering drugs [6]. Linagliptin was also efficacious in reducing HbA1c and well tolerated, and appears to reduce the risk of hypoglycemia when added to basal insulin [53]. Improved glycemic control was achieved with saxagliptin as monotherapy, add-on therapy, or initial combination therapy with metformin in elderly T2DM patients [57]. The efficacy and safety of sitagliptin was confirmed in elderly T2DM patients (≥ 75 years) with improved HbA1c levels and no increased risk of hypoglycemia [60]. Treatment with vildagliptin achieved individualized glycemic target levels with no safety or tolerability issues in elderly T2DM patients [7] with significantly improved compliance and persistence to treatment and lower rates of hypoglycemic events [56].
The favorable safety profile of DPP-4 inhibitors, especially in terms of reduced risk of hypoglycemia, may also result in improved ease of use of these drugs, as compared with other glucose-lowering agents that require regular food intake to prevent hypoglycemic events. Improved ease of use is no doubt a major advantage, especially for elderly T2DM patients. Additional characteristics that may constitute an advantage over other antihyperglycemics in older diabetes patients, who are often frail and on polytherapy, include the lack of clinically meaningful drug interactions for most gliptins (Table 1), and a neutral or protective effect on the risk of bone fractures [11,12,13,14,15, 61, 62].
Real-world evidence
Real-world evidence is crucial for demonstrating the effectiveness of a novel drug, that is, its ability to work under real-life conditions and to provide benefits that are important for patients. Regulatory authorities and decision-makers, along with the evidence from RCTs, increasingly request it. The worldwide, real-life observational EDGE study was conducted in more than 45,000 patients from 27 countries to compare the effectiveness of vildagliptin in dual therapy with other commonly used glucose-lowering agents for second-line, mostly metformin–sulfonylureas or metformin–pioglitazone combinations [63]. Vildagliptin not only achieved a reduction in HbA1c of 1.19% over 12 months, comparable to that observed in RCTs, but was also superior to the comparators at every time point over the year. Similarly, a sub-analysis of the DARWIN-T2D real-world study [64] demonstrated significant benefits of DPP-4 inhibitors in terms of reductions in HbA1c, fasting glucose, and body weight compared with the sulfonylurea, gliclazide, after a median of 6-month follow-up [32]. Together these studies highlight the importance of real-world evidence in enhancing our understanding of the potential role that therapeutic drugs have in clinical practice.
Following the introduction of the newer oral antihyperglycemics in 2008, the AIFA launched the Anti-diabetics Monitoring Registry to monitor the use of incretin-based therapies and for post-marketing safety surveillance [65]. Of 77,864 records entered between February 2008 and August 2010, 75,283 were of patients initiating a DPP-4 inhibitor or a GLP-1 receptor agonist (38,811 sitagliptin, 21,064 exenatide, and 17,989 vildagliptin). Patients aged ≥ 75 years were approximately 10% of the patients treated with a DPP-4 inhibitor. Reductions of HbA1c levels were − 0.88% and − 0.94% for sitagliptin and vildagliptin, respectively. The probability of achieving the HbA1c target of 7% (53 mmol/mol) with both gliptins decreased rapidly with increasing baseline HbA1c. Body weight decreased with treatment by approximately 1%. The safety profile of sitagliptin and vildagliptin was similar to that reported in RCTs.
Safety of DPP-4 inhibitors
Overall, a favorable safety and tolerability profile of DPP-4 inhibitors has emerged from RCTs [27]. Indeed, the incidence of hypoglycemia was found to be lower with DPP-4 inhibitors compared with sulfonylureas but was not significantly different compared with placebo or GLP-1 receptor agonists in an evaluation of 63 systematic reviews of DPP-4 inhibitors for the treatment of T2DM [44]. DPP-4 inhibitors also had similar effects in reducing the incidence of CV events, CV mortality, infections, diarrhea and nausea compared with placebo, but were associated with an lower risk of CV events compared with sulfonylureas treatment [44]. Similarly, DPP-4 inhibitors were also associated with no increase in the incidence of adverse events in a Bayesian network meta-analysis of 58 randomized controlled trials, although vildagliptin 100 mg once daily and sitagliptin 100 mg once daily were associated with the lowest probability in reducing the incidence of hypoglycemia and upper respiratory tract infection, respectively [45].
This favorable safety and tolerability profile has been largely confirmed in vulnerable D2TM subpopulations, such as elderly patients and patients with renal impairment, as well as in the real-world observational and the post-marketing setting [27, 63, 66, 67]. Table 3 provides an overview of the safety profile of the five available gliptins that has emerged from clinical trials and post-marketing surveillance.
Over the past decade, new regulatory guidelines have enforced the adjudication of all CV events when testing novel diabetes drugs. Endpoints of CV mortality, myocardial infarction, stroke, and hospitalization for heart failure were, therefore, included in most clinical studies on DPP-4 inhibitors. Five large, placebo-controlled, CV safety outcome trials in T2DM patients with established CV disease were conducted with saxagliptin (SAVOR-TIMI 53 trial, ~ 16,500 patients), alogliptin (EXAMINE trial, ~ 5400 patients), sitagliptin (TECOS trial, ~ 15,000 patients), and linagliptin (CARMELINA trial, 6,979 patients; CAROLINA trial, ~ 6000 patients) (Table 3) [68,69,70,71,72]. The EXAMINE trial revealed the non-inferiority of alogliptin vs placebo in terms of CV events [71]. Findings from the SAVOR-TIMI 53 trial confirmed the overall CV safety of saxagliptin, but raised a warning related to the increase in the risk of hospitalization for heart failure in the saxagliptin group [70]. No CV safety issues emerged from the TECOS trial evaluating sitagliptin [68]. The CARMELINA trial demonstrated a non-inferior risk of a composite CV outcome over a median of 2.2 years for linagliptin vs placebo when added to standard care in T2DM patients with established CV and/or kidney complications [72]. The results concerning the CV outcomes associated with linagliptin (CAROLINA trial) are expected soon. No CV outcome trial was planned for vildagliptin, but several trials have addressed its CV safety [73, 74]. In a meta-analysis (40 trials, 17,446 patients), vildagliptin was not associated with an increased risk of adjudicated major adverse CV events vs the comparators (any non-vildagliptin agent) (Table 3) [73]. The analysis did not find a significant increased risk of heart failure in patients treated with vildagliptin. The VIVIDD trial evaluated the effect of a 52-week treatment with vildagliptin on left ventricular ejection fraction (LVEF) in 254 subjects with T2DM and congestive heart failure [74]. The results showed no major effect on LVEF, but a statistically significant increase in left ventricular end-diastolic volume in the vildagliptin arm vs the placebo arm. The clinical implications of this observation are currently unclear. Based on the available evidence on CV outcomes associated with DPP-4 inhibitors, the general consensus in the field is that more data are needed for most gliptins, notably in patients with established heart failure and left ventricular systolic dysfunction [43, 74, 75].
Patients with T2DM have a twofold increased risk of developing acute pancreatitis compared with healthy individuals [67]. All incretin-based therapies have been extensively evaluated for pancreatic safety, due to the potential risk of pancreatic events, although no conclusive evidence of a causal relationship is available. Although an extensive evaluation of non-clinical and clinical data by the European Medicines Agency and the US Food and Drug Administration suggested no causal association between incretin-based therapies and pancreatic adverse events, the risk of acute pancreatitis has been added to the label of all DPP-4 inhibitors (Table 1) [76]. Notably, similar rates of acute or chronic pancreatitis have been reported in four large, placebo-controlled, CV safety outcome trials in T2DM patients with established CV disease: EXAMINE (alogliptin), CARMELINA (linagliptin), SAVOR-TIMI (saxagliptin), and TECOS (sitagliptin) (Table 3) [68, 70,71,72].
Results concerning pancreatic safety obtained in meta-analyses are conflicting [77]. A meta-analysis, including the large CV outcome trials discussed above, demonstrated an increased relative risk of acute pancreatitis (odds ratio 1.79; 95% CI 1.13–2.82) versus placebo, although the absolute risk increase was low (0.13%) [78]. A recent meta-analysis of 38 trials (59,404 patients), which compared DPP-4 inhibitors with placebo or other antihyperglycemic agents, found an increased risk of acute pancreatitis (Peto odds ratio 1.72, 95% CI 1.18–2.53) with DPP-4 inhibitors, but no relationship with pancreatic cancer [77]. The estimated number needed to harm was 1066, confirming that the risk of developing acute pancreatitis is low [77,78,79].
Conclusions and perspectives
Ten years of use in clinical practice, along with the evidence from an extensive program of clinical research and post-marketing surveillance, show that the development of DPP-4 inhibitors has been a valuable addition to the available therapies for T2DM. DPP-4 inhibitors, which stimulate insulin secretion in a glucose-dependent manner, are characterized by a gradual and durable lowering effect on HbA1c levels. Compared with commonly used oral glucose-lowering medications, DPP-4 inhibitors are associated with a low risk of hypoglycemic events and body weight increase. Due to the favorable safety and tolerability, DPP-4 inhibitors are indicated for the treatment of patients at high risk of hypoglycemia, including older patients and patients with renal impairment. They can be added at any stage of the treatment plan, with no major clinical barriers to their prescription, and are easy to administer.
With regard to diabetes care in Italy, evidence from national registries and databases suggests that DPP-4 inhibitors are underused, and that sulfonylureas and glinides continue to be the first choice when glycemic control with metformin monotherapy needs to be intensified. The prescribing limitations issued by the AIFA for incretin-based therapies are among the reasons for the underuse of DPP-4 inhibitors. According to these limitations, incretin-based therapies are reimbursed only if prescribed by a diabetes specialist; furthermore they cannot be prescribed to patients with HbA1c < 7.5% (< 58 mmol/mol) even if the current medication has a less favorable risk–benefit profile, or to patients with HbA1c > 8.5–9.0% (> 69–75 mmol/mol). A loosening of the prescribing limitations would be desirable in Italy to improve access to appropriate treatment, especially for patients, such as older individuals with CKD and other comorbidities, who have very few treatment options besides DPP-4 inhibitors. Such a change will require educational efforts directed to GPs to train them in the use of the newer glucose-lowering therapies.
Further research on DPP-4 inhibitors is needed. Important safety issues, including CV outcomes and the risk of acute pancreatitis, are still under investigation and need to be conclusively established. Clinical research in this area is also ongoing and will hopefully improve our understanding of central questions on the pathophysiology of diabetes.
References
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98. https://doi.org/10.1038/nrendo.2017.151
ARNO (2017) Osservatorio ARNO Diabete CINECA-SID. Il profilo assistenziale della popolazione con diabete. Rapporto 2017 (Observatory ARNO Diabetes CINECA-SID. The care profile of the population with diabetes. Report 2017). http://arno.cineca.it/journal/wp-content/uploads/2017/10/Diabete_2017_con-copertina.pdf. Accessed 1 August 2018
SID-SIGG (2017) Società Italiana di Diabetologia (SID) e Società Italiana di Gerontologia e Geriatria (SIGG). Position statement—Personalizzazione del trattamento dell’iperglicemia nell’anziano con diabete tipo 2 (Italian Society of Diabetology [SID] and Italian Society of Gerontology and Geriatrics [SIGG]. Position statement—customization of the treatment of hyperglycemia in the elderly with type 2 diabetes). https://www.sigg.it/wp-content/uploads/2017/11/SID-SIGG-Documento-ufficiale.pdf. Accessed 1 Aug 2018
Soe K, Sacerdote A, Karam J, Bahtiyar G (2011) Management of type 2 diabetes mellitus in the elderly. Maturitas 70(2):151–159. https://doi.org/10.1016/j.maturitas.2011.07.006
Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ (2013) Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet 382(9902):1413–1423. https://doi.org/10.1016/S0140-6736(13)61500-7
Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldanius PM (2013) Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet 382(9890):409–416. https://doi.org/10.1016/S0140-6736(13)60995-2
Strain WD, Paldanius PM (2017) Dipeptidyl peptidase-4 inhibitor development and post-authorisation programme for vildagliptin—clinical evidence for optimised management of chronic diseases beyond type 2 diabetes. Eur Endocrinol 13(2):62–67. https://doi.org/10.17925/EE.2017.13.02.62
Deacon CF (2018) Peptide degradation and the role of DPP-4 inhibitors in the treatment of type 2 diabetes. Peptides 100:150–157. https://doi.org/10.1016/j.peptides.2017.10.011
Scheen AJ (2012) DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab 38(2):89–101. https://doi.org/10.1016/j.diabet.2011.11.001
Vildagliptin. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf. Accessed 1 Aug 2018
Sitagliptin. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000722/WC500039054.pdf. Accessed 1 Aug 2018
Saxagliptin. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001039/WC500044316.pdf. Accessed 1 Aug 2018
Linagliptin. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002110/WC500115745.pdf. Accessed 1 Aug 2018
Alogliptin. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002182/WC500152271.pdf. Accessed 1 Aug 2018
AMD-SID (2018) Associazione Medici Diabetologi (AMD)—Società Italiana di Diabetologia (SID). Standard italiani per la cura del diabete mellito (Doctors Diabetologists Association [AMD]—Italian Society of Diabetology [SID]. Italian standards for the treatment of diabetes mellitus). http://www.siditalia.it/pdf/Standard%20di%20Cura%20AMD%20-%20SID%202018_protetto.pdf. Accessed 1 Aug 2018
American Diabetes Association (2017) Standards of medical care in diabetes—2017. Diabetes Care 40(Supplement 1):S1–S135. http://care.diabetesjournals.org/content/diacare/suppl/2016/12/15/40.Supplement_1.DC1/DC_40_S1_final.pdf. Accessed 1 Aug 2017
ISTAT (2017) Il diabete in Italia. Anni 2000–2016 (Diabetes in Italy. Years 2000–2016). https://www.istat.it/it/files//2017/07/REPORT_DIABETE.pdf. Accessed 1 Aug 2018
Pugliese G, Solini A, Bonora E, Fondelli C, Orsi E, Nicolucci A et al (2014) Chronic kidney disease in type 2 diabetes: lessons from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicentre Study. Nutr Metab Cardiovasc Dis 24(8):815–822. https://doi.org/10.1016/j.numecd.2014.02.013
Baviera M, Avanzini F, Marzona I, Tettamanti M, Vannini T, Cortesi L et al (2017) Cardiovascular complications and drug prescriptions in subjects with and without diabetes in a Northern region of Italy, in 2002 and 2012. Nutr Metab Cardiovasc Dis 27(1):54–62. https://doi.org/10.1016/j.numecd.2016.10.006
The Medicines Utilization Monitoring Centre (2017) National Report on Medicine Use in Italy 2016. AIFA, Italian Medicine Agency, Rome. http://www.agenziafarmaco.gov.it. Accessed 1 Aug 2018
AIFA (2015) Piano Terapeutico per la prescrizione di Incretine/inibitori DPP-4 nel trattamento del diabete tipo 2 (aggiornamento marzo 2015) (Therapeutic Plan for the prescription of Incretine / DPP-4 inhibitors in the treatment of type 2 diabetes [update March 2015]). http://www.siditalia.it/images/Documenti/NEWS/Nuovo_piano_terapeutico_incretine.pdf. Accessed 1 Aug 2018
Niskanen L, Hahl J, Haukka J, Leppa E, Miettinen T, Mushnikov V et al (2018) Type 2 diabetes and treatment intensification in primary care in Finland. Acta Diabetol 55(11):1171–1179. https://doi.org/10.1007/s00592-018-1199-7
ARNO (2015) Società Italiana di Diabetologia. Osservatorio ARNO Diabete Anziani. Il profilo assistenziale della popolazione anziana con diabete Elaborazioni su dati anno 2015 (a cura di Cineca-SID) (Italian Society of Diabetology. ARNO Observatory for Elderly Diabetes. The care profile of the elderly population with diabetes. Elaborations on data for 2015 [edited by Cineca-SID]). http://arno.cineca.it/portal/wp-content/uploads/2017/04/2017-Osservatorio-ARNO-Diabete-Anziani.pdf. Accessed 1 Aug 2018
Solini A, Penno G, Bonora E, Fondelli C, Orsi E, Trevisan R et al (2013) Age, renal dysfunction, cardiovascular disease, and antihyperglycemic treatment in type 2 diabetes mellitus: findings from the Renal Insufficiency and Cardiovascular Events Italian Multicenter Study. J Am Geriatr Soc 61(8):1253–1261. https://doi.org/10.1111/jgs.12381
Ahren B, Mathieu C, Bader G, Schweizer A, Foley JE (2014) Efficacy of vildagliptin versus sulfonylureas as add-on therapy to metformin: comparison of results from randomised controlled and observational studies. Diabetologia 57(7):1304–1307. https://doi.org/10.1007/s00125-014-3222-z
Scheen AJ (2018) The safety of gliptins: updated data in 2018. Expert Opin Drug Saf 17(4):387–405. https://doi.org/10.1080/14740338.2018.1444027
Zhang Y, Hong J, Chi J, Gu W, Ning G, Wang W (2014) Head-to-head comparison of dipeptidyl peptidase-IV inhibitors and sulfonylureas—a meta-analysis from randomized clinical trials. Diabetes Metab Res Rev 30(3):241–256. https://doi.org/10.1002/dmrr.2482
Zhou JB, Bai L, Wang Y, Yang JK (2016) The benefits and risks of DPP4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: accumulated evidence from randomised controlled trial. Int J Clin Pract 70(2):132–141. https://doi.org/10.1111/ijcp.12761
Trevisan R (2017) The role of vildagliptin in the therapy of type 2 diabetic patients with renal dysfunction. Diabetes Ther 8(6):1215–1226. https://doi.org/10.1007/s13300-017-0302-3
Brath H, Paldanius PM, Bader G, Mathieu C (2017) Relationship between duration of type 2 diabetes and effectiveness of DPP-4 inhibitor versus sulfonylurea as add-on therapy: a post hoc analysis. Diabetes Ther 8(4):829–836. https://doi.org/10.1007/s13300-017-0276-1
Fadini GP, Bottigliengo D, D’Angelo F, Cavalot F, Bossi AC, Zatti G et al (2018) Comparative effectiveness of DPP-4 inhibitors versus sulfonylurea for the treatment of type 2 diabetes in routine clinical practice: a retrospective multicenter real-world study. Diabetes Ther 9(4):1477–1490. https://doi.org/10.1007/s13300-018-0452-y
Buzzetti R, Pozzilli P, Frederich R, Iqbal N, Hirshberg B (2016) Saxagliptin improves glycaemic control and C-peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Metab Res Rev 32(3):289–296. https://doi.org/10.1002/dmrr.2717
D’Alessio DA, Denney AM, Hermiller LM, Prigeon RL, Martin JM, Tharp WG et al (2009) Treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetes. J Clin Endocrinol Metab 94(1):81–88. https://doi.org/10.1210/jc.2008-1135
Utzschneider KM, Tong J, Montgomery B, Udayasankar J, Gerchman F, Marcovina SM et al (2008) The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care 31(1):108–113. https://doi.org/10.2337/dc07-1441
Zhao Y, Yang L, Xiang Y, Liu L, Huang G, Long Z et al (2014) Dipeptidyl peptidase 4 inhibitor sitagliptin maintains beta-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab 99(5):E876–E880. https://doi.org/10.1210/jc.2013-3633
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M et al (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38(1):140–149. https://doi.org/10.2337/dc14-2441
Deacon CF, Mannucci E, Ahren B (2012) Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab 14(8):762–767. https://doi.org/10.1111/j.1463-1326.2012.01603.x
Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D (2014) Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of long-term randomised controlled trials. BMJ Open 4(6):e005442. https://doi.org/10.1136/bmjopen-2014-005442
Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, Ceriello A, Giugliano D (2011) Dipeptidyl peptidase-4 inhibitors and HbA1c target of < 7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab 13(7):594–603. https://doi.org/10.1111/j.1463-1326.2011.01380.x
Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A (2012) Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 344:e1369. https://doi.org/10.1136/bmj.e1369
Mishriky BM, Cummings DM, Tanenberg RJ (2015) The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 109(2):378–388. https://doi.org/10.1016/j.diabres.2015.05.025
Wu D, Li L, Liu C (2014) Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab 16(1):30–37. https://doi.org/10.1111/dom.12174
Ling J, Ge L, Zhang DH, Wang YF, Xie ZL, Tian JH et al (2018) DPP-4 inhibitors for the treatment of type 2 diabetes: a methodology overview of systematic reviews. Acta Diabetol. https://doi.org/10.1007/s00592-018-1164-5 (Epub ahead of print)
Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH et al (2018) The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials. Acta Diabetol. https://doi.org/10.1007/s00592-018-1222-z (Epub ahead of print)
Esposito K, Chiodini P, Capuano A, Maiorino MI, Bellastella G, Giugliano D (2014) Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors: meta-regression analysis of 78 randomized controlled trials with 20,053 patients. Endocrine 46(1):43–51. https://doi.org/10.1007/s12020-013-0090-0
Cheng D, Fei Y, Liu Y, Li J, Chen Y, Wang X, Wang N (2014) Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS One 9(10):e111543. https://doi.org/10.1371/journal.pone.0111543
Howse PM, Chibrikova LN, Twells LK, Barrett BJ, Gamble JM (2016) Safety and efficacy of incretin-based therapies in patients with type 2 diabetes mellitus and CKD: a systematic review and meta-analysis. Am J Kidney Dis 68(5):733–742. https://doi.org/10.1053/j.ajkd.2016.06.014
Li R, Wang R, Li H, Sun S, Zou M, Cheng G (2016) Short-term and long-term effects of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with renal impairment: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 32(6):460–469. https://doi.org/10.1002/dmrr.2731
Singh-Franco D, Harrington C, Tellez-Corrales E (2016) An updated systematic review and meta-analysis on the efficacy and tolerability of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes with moderate to severe chronic kidney disease. SAGE Open Med 4:1–16. https://doi.org/10.1177/2050312116659090
Thomas MC, Paldanius PM, Ayyagari R, Ong SH, Groop PH (2016) Systematic literature review of DPP-4 inhibitors in patients with type 2 diabetes mellitus and renal impairment. Diabetes Ther 7(3):439–454. https://doi.org/10.1007/s13300-016-0189-4
Halimi S, Raccah D, Schweizer A, Dejager S (2010) Role of vildagliptin in managing type 2 diabetes mellitus in the elderly. Curr Med Res Opin 26(7):1647–1656. https://doi.org/10.1185/03007995.2010.485881
Inzucchi SE, Nauck MA, Hehnke U, Woerle HJ, von Eynatten M, Henry RR (2015) Improved glucose control with reduced hypoglycaemic risk when linagliptin is added to basal insulin in elderly patients with type 2 diabetes. Diabetes Obes Metab 17(9):868–877. https://doi.org/10.1111/dom.12490
Schweizer A, Dejager S (2013) Experience with vildagliptin in patients>/=75 years with type 2 diabetes and moderate or severe renal impairment. Diabetes Ther 4(2):257–267. https://doi.org/10.1007/s13300-013-0027-x
Schweizer A, Dejager S, Foley JE, Shao Q, Kothny W (2011) Clinical experience with vildagliptin in the management of type 2 diabetes in a patient population>/=75 years: a pooled analysis from a database of clinical trials. Diabetes Obes Metab 13(1):55–64. https://doi.org/10.1111/j.1463-1326.2010.01325.x
Sicras-Mainar A, Navarro-Artieda R (2014) Use of metformin and vildagliptin for treatment of type 2 diabetes in the elderly. Drug Des Devel Ther 8:811–818. https://doi.org/10.2147/DDDT.S65327
Karyekar CS, Ravichandran S, Allen E, Fleming D, Frederich R (2013) Tolerability and efficacy of glycemic control with saxagliptin in older patients (aged>/= 65 years) with inadequately controlled type 2 diabetes mellitus. Clin Interv Aging 8:419–430. https://doi.org/10.2147/CIA.S41246
Pratley RE, McCall T, Fleck PR, Wilson CA, Mekki Q (2009) Alogliptin use in elderly people: a pooled analysis from phase 2 and 3 studies. J Am Geriatr Soc 57(11):2011–2019. https://doi.org/10.1111/j.1532-5415.2009.02484.x
Rosenstock J, Wilson C, Fleck P (2013) Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab 15(10):906–914. https://doi.org/10.1111/dom.12102
Umezawa S, Kubota A, Maeda H, Kanamori A, Matoba K, Jin Y et al (2015) Two-year assessment of the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes: Post hoc analysis of the ASSET-K study. BMC Endocr Disord 15:34. https://doi.org/10.1186/s12902-015-0033-2
Fu J, Zhu J, Hao Y, Guo C, Zhou Z (2016) Dipeptidyl peptidase-4 inhibitors and fracture risk: an updated meta-analysis of randomized clinical trials. Sci Rep 6:29104. https://doi.org/10.1038/srep29104
Yang J, Huang C, Wu S, Xu Y, Cai T, Chai S et al (2017) The effects of dipeptidyl peptidase-4 inhibitors on bone fracture among patients with type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. PLoS One 12(12):e0187537. https://doi.org/10.1371/journal.pone.0187537
Mathieu C, Barnett AH, Brath H, Conget I, de Castro JJ, Goke R et al (2013) Effectiveness and tolerability of second-line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real-life worldwide observational study (EDGE). Int J Clin Pract 67(10):947–956. https://doi.org/10.1111/ijcp.12252
Fadini GP, Zatti G, Baldi I, Bottigliengo D, Consoli A, Giaccari A et al (2018) Use and effectiveness of dapagliflozin in routine clinical practice: an Italian multicentre retrospective study. Diabetes Obes Metab 20(7):1781–1786. https://doi.org/10.1111/dom.13280
Montilla S, Marchesini G, Sammarco A, Trotta MP, Siviero PD, Tomino C et al (2014) Drug utilization, safety, and effectiveness of exenatide, sitagliptin, and vildagliptin for type 2 diabetes in the real world: data from the Italian AIFA Anti-diabetics Monitoring Registry. Nutr Metab Cardiovasc Dis 24(12):1346–1353. https://doi.org/10.1016/j.numecd.2014.07.014
Gokhale M, Buse JB, Jonsson Funk M, Lund J, Pate V, Simpson RJ, Sturmer T (2017) No increased risk of cardiovascular events in older adults initiating dipeptidyl peptidase-4 inhibitors vs therapeutic alternatives. Diabetes Obes Metab 19(7):970–978. https://doi.org/10.1111/dom.12906
Mathieu C, Kozlovski P, Paldanius PM, Foley JE, Modgill V, Evans M, Serban C (2017) Clinical safety and tolerability of vildagliptin—insights from randomised trials, observational studies and post-marketing surveillance. Eur Endocrinol 13(2):68–72. https://doi.org/10.17925/EE.2017.13.02.68
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J et al (2015) Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 373(3):232–242. https://doi.org/10.1056/NEJMoa1501352
Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM et al (2015) Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diab Vasc Dis Res 12(3):164–174. https://doi.org/10.1177/1479164115570301
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B et al (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369(14):1317–1326. https://doi.org/10.1056/NEJMoa1307684
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL et al (2013) Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369(14):1327–1335. https://doi.org/10.1056/NEJMoa1305889
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N et al (2018) Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. https://doi.org/10.1001/jama.2018.18269
McInnes G, Evans M, Del Prato S, Stumvoll M, Schweizer A, Lukashevich V et al (2015) Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17,000 patients. Diabetes Obes Metab 17(11):1085–1092. https://doi.org/10.1111/dom.12548
McMurray JJV, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W et al (2018) Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail 6(1):8–17. https://doi.org/10.1016/j.jchf.2017.08.004
Bonora E, Cigolini M (2016) DPP-4 inhibitors and cardiovascular disease in type 2 diabetes mellitus. Expectations, observations and perspectives. Nutr Metab Cardiovasc Dis 26(4):273–284. https://doi.org/10.1016/j.numecd.2016.03.002
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C (2014) Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 370(9):794–797. https://doi.org/10.1056/NEJMp1314078
Pinto LC, Rados DV, Barkan SS, Leitao CB, Gross JL (2018) Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: a meta-analysis with trial sequential analysis. Sci Rep 8(1):782. https://doi.org/10.1038/s41598-017-19055-6
Tkac I, Raz I (2017) Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care 40(2):284–286. https://doi.org/10.2337/dc15-1707
Abbas AS, Dehbi HM, Ray KK (2016) Cardiovascular and non-cardiovascular safety of dipeptidyl peptidase-4 inhibition: a meta-analysis of randomized controlled cardiovascular outcome trials. Diabetes Obes Metab 18(3):295–299. https://doi.org/10.1111/dom.12595
Acknowledgements
We thank Lorenza Lanini, an independent medical writer, and Melanie Gatt (PhD), who provided editorial assistance, on behalf of Springer Healthcare Communications. This support was funded by Novartis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Giorgio Sesti has received speaker/consulting honoraria from Novo Nordisk, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Merck, Novartis, Sanofi, Amgem, GlaxoSmithKline, Mylan, Abbott, and Servier. Angelo Avogaro has received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Bayer, Boeringher-Ingelheim, Sanofi, Mediolanum, Janssen, NovoNordisk, Eli Lilly, Servier, Vifor Pharma, Jannsen, and Takeda. Sara Belcastro is a consultant for Novo nordisk, Mundipharma, Novartis, and Merck & co. Simona Frontoni has received honoraria for participating in advisory boards or speaking engagement from all companies producing medicine for diabetes care over the last 10 years. Her institution received research grant from Novo Nordisk, MSD, Ibsa. Cecilia Invitti has received a speaker honorarium from Guidotti. Benedetta Maria Bonora, Marina Croci, Giuseppe Daniele, Marco Dauriz, Francesco Dotta, Caterina Formichi, Emanuela Orsi, Fabiana Picconi and Veronica Resi declare that they have no conflict of interest. Enzo Bonora has received honoraria for participating in advisory boards or speaking engagement from all companies producing medicine for diabetes care over the last 10 years. His institution received research grant from Novo Nordisk, Takeda, AstraZeneca. Francesco Purrello has received honoraria for participating in advisory boards or speaking engagement from Eli Lilly, Novo Nordisk, Sanofi, MSD, AstraZeneca, Boheringer, GSK, Novartis, Menarini. His institution received research grant from Eli Lilly, Novo Nordisk, AstraZeneca.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Managed by Massimo Federici.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sesti, G., Avogaro, A., Belcastro, S. et al. Ten years of experience with DPP-4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol 56, 605–617 (2019). https://doi.org/10.1007/s00592-018-1271-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1271-3