Abstract
Aims
Interleukin-8 (IL-8) is a chemokine involved in systemic immunity, macrophages infiltration and activation in adipose tissue and may play a significant role in the pathogenesis of type 2 diabetes (T2D) and atherosclerosis. Aims of this study were to evaluate circulating IL-8 levels in adult patients with T2D in comparison with non-diabetic subjects and to describe clinical and biochemical correlates of IL-8 concentration.
Methods
For this cross-sectional study, we enrolled 79 consecutive T2D individuals referring to our diabetes outpatient clinics at Sapienza University of Rome, and 37 sex, age and BMI comparable non-diabetic subjects as a control group. Clinical parameters and medical history were recorded; fasting blood sampling was performed for biochemistry and for measuring serum IL-8, IL-6, TNF-α, CRP, adiponectin and 25(OH)vitamin D [25(OH)D] levels.
Results
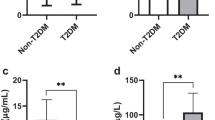
Patients with T2D exhibited significantly higher serum IL-8 levels than non-diabetic subjects (69.27 ± 112.83 vs. 16.03 ± 24.27 pg/mL, p < 0.001). In diabetic patients, increased IL-8 concentration correlated with higher IL-6 (p < 0.001), TNF-α (p = 0.02), FBG (p = 0.035), HbA1c (p = 0.04) and LDL-C (p = 0.04) and with lower adiponectin (p = 0.02) and 25(OH)D (p = 0.003) concentrations.
Conclusions
Patients with T2D display a marked elevation of circulating IL-8 levels which identify subjects with worse inflammatory, glycometabolic and lipid profile and lower vitamin D levels. Further studies are warranted for evaluating a possible role of IL-8 as a novel marker for risk stratification in T2D patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2D) is characterized by a condition of systemic low-grade inflammation which represents a key factor for the development of insulin resistance and associated comorbidities, such as non-alcoholic fatty liver disease (NAFLD) and atherosclerosis [1,2,3,4,5,6,7]. In this scenario, adipose tissue (AT) acts as an endocrine and immune organ by secreting many bioactive peptides and, thus, influencing insulin action. Advances in obesity research have led to the recognition that insulin resistance is tightly connected with immunity. In condition of obesity, AT undergoes infiltration and activation of immune cells and, mostly, macrophages, which represent a major source of inflammatory mediators [8] and trigger, in turn, the development of AT-insulin resistance [9, 10].
Indeed, the coexistence of systemic and AT-associated chronic inflammation and impaired insulin sensitivity lay the basis for the development of T2D [11].
Several cross-sectional studies showed that insulin resistance and T2D are associated with higher circulating levels of C-reactive protein (CRP), IL-6 and TNF-α [12,13,14,15,16,17]; in addition, the chemokine system, in particular interleukin-8 (IL-8), came more recently into the focus of metabolic inflammation research [18,19,20].
IL-8 is a pro-inflammatory polypeptide belonging to the CXC chemokine superfamily, characterized by the presence of two cysteine residues separated by an intervening aminoacid in the first three positions [21], and is secreted by several cell types, including adipocytes, monocytes/macrophages, T-lymphocytes, endothelial and epidermal cells [22, 23]. As a multifunctional chemokine, it has chemoattractant and mitogenic effects on neutrophils [24], as well as on T-cells, vascular smooth muscle cells, vascular endothelial cells and monocytes [25,26,27,28].
Several studies have described an association between IL-8 and hepatic metabolic pathways in humans. In particular, IL-8 was shown to be produced by hepatocytes under the stimulus of free fatty acids via the activation of NF-kB and JNK pathways [29]. Moreover, IL-8 secretion significantly increased in condition of chronic liver diseases, suggesting a role for this cytokine in the recruitment and activation of hepatic macrophages [30]. Indeed, several studies demonstrated a role of IL-8 in pathogenesis and progression of NAFLD [31,32,33,34,35,36,37].
Among its multiple actions, IL-8 also promotes macrophages infiltration in AT [38], inducing local and systemic inflammation and representing, in turn, a potential link between AT dysfunction and insulin resistance-related conditions [31,32,33,34,35,36,37,38,39,40].
Data showed that IL-8 enhanced the IL-8 mRNA expression in human adipocytes, and adipocytes expressed the main receptor for IL-8, playing, thus, an autocrine effect on these cells [41]. Furthermore, Kobashi et al. [41] demonstrated that the inflammatory stimulation creates a vicious circle of IL-8 production in human adipocytes via ERK pathway and/or p38 MAPK pathway. Taken together, these data suggest that IL-8 plays an important role in both the initiation and the maintenance of inflammation in the AT. Additionally, in vitro studies showed that IL-8 induced insulin resistance via the inhibition of insulin-induced Akt phosphorylation in human adipocytes and suggested that the attenuation of IL-8 production and/or action may represent a target for the prevention of diabetes and its complications. These results support the hypothesis of a central role of IL-8 in influencing adipocyte physiology beyond its traditional function in the recruitment of inflammatory cells, but despite the bulk of data on IL-8 involvement in cardiometabolic diseases, studies specifically designed for assessing the role of IL-8 in diabetes are lacking. Therefore, aims of this study were to evaluate circulating IL-8 levels in adult patients with T2D in comparison with non-diabetic subjects and to describe clinical and biochemical correlates of IL-8 concentration.
Methods
For our purposes, we enrolled 79 patients with T2D (M/F: 45/34, age: 58 ± 9 years, BMI: 33.65 ± 6.3 kg/m2, T2D duration: 7.1 ± 6.3 years) among those referring to our diabetes outpatient clinics at Sapienza University of Rome, and 37 non-diabetic subjects comparable for sex, age and BMI (M/F: 20/17, age: 54 ± 17 years, BMI: 34.29 ± 4.4 kg/m2, p-values not significant) not affected by any comorbidities and not treated with any medications at the time of study recruitment, as a control group.
Each subject underwent medical history collection, physical examination, with measurement of height, weight, waist circumference, systolic and diastolic blood pressure (SBP, DBP; mmHg), hepatic ultrasound (US) for the evaluation of NAFLD, and blood sampling for assessing biochemistry and immune-inflammatory profile. Fasting blood glucose (FBG, mg/dL), glycosylated hemoglobin (HbA1c, %–mmol/mol), total cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), blood ureic nitrogen (BUN, mg/dL), creatinine (mg/dL), aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), alkaline phosphatase (ALP, IU/L) and gamma-glutamyl transpeptidase (γ-GT, IU/L) were measured by standard laboratory methods. LDL-cholesterol (mg/dL) was obtained using the Friedwald formula. Fasting plasma insulin levels were measured by radio-immuno-assay (μU/L, ADVIA Insulin Ready Pack 100, Bayer Diagnostics, Italy); indexes of insulin resistance (HOMA-IR) and secretion (HOMA-β%) have been calculated as reported elsewhere [42].
Serum IL-8, IL-6 and TNF-α levels (pg/mL) were measured by BioPlex Multiplex Immunoassays Biorad on sera frozen immediately after separation and stored at −25 °C for few days. Circulating adiponectin concentration was assessed by immunoenzymatic assay (Phoenix Pharmaceutical, Inc.) and CRP by high-sensitivity CRP assay (mg/dL, latex-enhanced immunoturbidimetric).
Among molecules with immunomodulation properties, we assessed systemic levels of vitamin D by dosing serum concentration of 25(OH)vitamin D [25(OH)D], the most stable form of this hormone, by colorimetric method (LIAISON, DiaSorin).
Statistics
SPSS version 17 statistical package was used to perform all the analyses. Student’s T test for continuous variables and χ 2 test for categorical variables were performed to compare mean values between two independent groups. Skewed variables underwent logarithmic transformation before the analyses. Correlations between continuous variables were calculated by Pearson’s coefficient, whereas Spearman’s coefficient was used for dichotomic/ordinal parameters. Correlation coefficients are reported as r values in the text. Bivariate and multivariate linear regression models were used to detect the association between serum IL-8, considered as a continuous variable, and all possible determinants. This is the first study specifically designed to investigate IL-8 profile in subjects with T2D in relation to the inflammatory and metabolic profile, therefore in order to calculate the study power we performed a post hoc sample size calculation showing that based on the two groups IL-8 mean and common standard deviation, a minimum of 23 subjects per group were needed for obtaining statistically significant results with α-error = 0.05 and power = 0.80.
Data are shown as mean ± standard deviation (SD) in both text and tables. A p-value <0.05 was considered statistically significant.
Results
Patients with T2D had significantly higher serum IL-8 levels than non-diabetic subjects (mean ± SD: 69.27 ± 112.83 pg/mL vs. 16.03 ± 24.27 pg/mL, p < 0.001); of note, diabetic patients also displayed higher CRP levels, total cholesterol, transaminases, SBP and lower adiponectin concentration than control group. Clinical and biochemical characteristics of study populations are summarized in Table 1.
In T2D patients, increased serum IL-8 levels correlated with higher IL-6 (r = 0.33, p < 0.001) and TNF-α (r = 0.22, p = 0.02). The positive association between IL-8 and TNF-α applied also to non-diabetic controls (r = 0.46, p = 0.005). Furthermore, in the diabetes cohort, higher IL-8 correlated with worse glycemic control (FBG, r = 0.22, p = 0.035; HbA1c, r = 0.25, p = 0.04) and lipid profile (LDL, r = 0.23, p = 0.04) and with lower serum concentration of adiponectin (r = −0.29, p = 0.02) and 25(OH)D (r = −0.35, p = 0.003). By contrast, no association was found between serum IL-8 and either parameters of total body adiposity, such as obesity, BMI and waist circumference, or systemic insulin resistance/secretion, as expressed by HOMA-IR and HOMA-β%. Moreover, no correlation was found between circulating IL-8 levels and the presence of US-detected NAFLD and serum transaminases. In total, 72% of T2D patients had systemic blood hypertension, 87% non-alcoholic fatty liver disease (NAFLD), 52% dyslipidemia, 56% obesity and 5% suffered from ischemic cardiopathy. No association was found between comorbidities and serum IL-8 levels in this population (hypertension: r = −0.09, p = 0.43; NAFLD: r = 0.03, p = 0.23; dyslipidemia: r = −0.13, p = 0.24; obesity: r = −0.02, p = 0.89; ischemic cardiopathy: r = −0.15, p = 0.23).
Within T2D subgroup, 85% of patients were treated with oral antidiabetic agents (OAD) and 17% with insulin, with or without ADO; 52% were also in treatment with statins. Thus, we performed bivariate correlation analyses showing no association between circulating IL-8 levels and therapies, both when considering just the presence of OAD treatment per se and when comparing different classes of OAD or insulin. Similarly, treatment with statins did not affect IL-8 in our population. Correlations among IL-8 concentration and clinical/biochemical parameters are shown in Table 2. For ascertaining the main determinant of increased IL-8 levels in the whole study population, we performed a multivariate linear regression analysis, showing that T2D was associated with higher IL-8 independently from the possible confounders (β: 0.52, p = 0.0001) (Table 3).
In addition, although higher CRP levels were observed in T2D patients compared to controls, CRP was not related to markers of increased risk profile, such as impaired glycemic control and lipid profile at the bivariate correlation analyses (data not shown). Furthermore, although higher CRP was associated with the presence of obesity (r = 0.28, p = 0.009), increased CRP levels did not identify a cytokine pattern suggestive of AT-related inflammation, showing no correlation with adiponectin (r = 0.025, p = 0.85), IL-6 (r = 0.16, p = 0.15) or TNF-α (r = −0.07, p = 0.51) concentration.
Discussion
Our study demonstrates that patients with T2D display significantly higher IL-8 levels than non-diabetic subjects which identify individuals with greater AT-associated inflammation, worse glycometabolic profile and low vitamin D levels.
Few previous studies investigated circulating IL-8 concentration in presence of diabetes, reporting inconclusive results. Zozulinska et al. [43] first found increased IL-8 levels in a small cohort of both type 1 and type 2 diabetic patients in poor glycemic control, in comparison with non-diabetic subjects. According to our results, they observed a linear correlation between higher IL-8 levels and HbA1c, but no data regarding systemic immune-inflammatory profile, clinical parameters such as adiposity and dysmetabolic conditions other than diabetes, were available in this study population [43].
Higher IL-8 levels in T2D, tightly associated with FBG, were also found in a report by Esposito et al. [44] examining a panel of inflammatory biomarkers in a cohort of 30 non-obese individuals with newly diagnosed T2D, naive for antidiabetic treatments. Contrarily, in a small intervention trial testing the effect of ticlopidine therapy on vascular complication in T2D, no difference was found in IL-8 levels between diabetic and non-diabetic individuals [45].
Interestingly, Samaras et al. [46] investigated SAT and VAT gene expression in individuals with or without T2D and demonstrated significantly higher IL-8 expression in VAT from T2D patients compared to VAT from non-diabetic controls. These data, along with our findings on increased circulating IL-8 levels in T2D subjects, support the hypothesis of a role for IL-8 as a marker of AT inflammation in presence of diabetes.
Of note, in our study population, IL-8 assessment showed high standard deviation and, as observed for other circulating molecules, several study participants reported undetectable serum IL-8 levels. However, unlike other cytokines, increased IL-8 concentration was able to identify a specific phenotype among patients with T2D, characterized by the presence of worse glycometabolic and inflammatory systemic profile.
This is the first study specifically designed to investigate IL-8 profile in subjects with T2D in relation to both systemic and AT-associated inflammation and clinical markers of metabolic diseases. Thus, beside showing the existence of a relationship between higher IL-8 and the presence of diabetes, our study demonstrated that, differently from what observed for a specific pro-inflammatory markers such as CRP, increased IL-8 levels are associated with unfavorable systemic inflammatory pattern—as expressed by higher IL-6 and TNF-α and lower adiponectin and active vitamin D levels—and worse glycemic and lipid profile.
In condition of chronic positive energy balance, AT is challenged to store large amounts of triglycerides and glucose-derived free fatty acids, leading to adipocytes expansion; however, once they are no longer able to increase in volume for safely storing nutrients, AT becomes dysfunctional, instigating FFAs spillover and releasing pro-inflammatory cytokines, thus contributing to AT-insulin resistance and chronic low-grade inflammation [47]. Indeed, in condition of visceral AT expansion, the main contributor to the development of AT inflammation is likely represented by the limited AT capacity to appropriately react to calories overload, as shown in non-obese subjects with T2D [48].
Notably, in this study no direct correlation was found between IL-8 and indicators of adiposity, such as BMI and waist circumference, likely showing that AT inflammation and impaired physiology, rather than obesity per se, may represent the key determinant of increased circulating IL-8 concentration in diabetes. This conclusion is reinforced by the evidence of an association between AT dysfunction and impaired IL-6, TNF-α and adiponectin concentrations [48,49,50] in dysmetabolic conditions. Thus, the above cytokines tightly associated with higher IL-8 levels in our study.
As to immune-inflammatory profile, we also observed an inverse association between IL-8 and circulating levels of vitamin D, known to display, beside its action on calcium and bone metabolism, a modulatory action on both innate and adaptive immunity [51, 52].
Since hypovitaminosis D is a risk factor for macrovascular complications in diabetes [53,54,55] and for all-cause and cardiovascular mortality in the general population [56], it is plausible to hypothesize that increased IL-8 levels and impaired vitamin D status may act “in synergy” determining worse metabolic risk profile in presence of diabetes. Thus, a preexisting condition of hypovitaminosis D and subsequent impaired immune regulation [51] could enhance the systemic low-grade inflammation underlying insulin resistance and diabetes with detrimental effects on cardiovascular outcomes.
Our study has some limitations. First, the cross-sectional design of this study does not allow to establish a certain causal relationship between increased IL-8 production and AT dysfunction, but we may speculate that, in T2D patients, higher circulating IL-8 levels represent and expression of AT inflammation, which, in turn, could lead to worse risk profile in diabetes. Second, the lack of AT histological data still considered the gold standard for identifying AT inflammation.
In conclusion, our study demonstrates that patients with T2D display a marked elevation of circulating IL-8 levels which identify subjects with worse inflammatory state and metabolic control independently from total adiposity and other possible confounders. Prospective studies will be needed to further evaluate the relevance of IL-8 in the disease process and to clarify whether it can be considered as a novel marker and a useful tool for risk stratification in T2D patients.
References
Paniagua JA (2016) Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes 7:483–514
Lopes HF, Corrêa-Giannella ML, Consolim-Colombo FM, Egan BM (2016) Visceral adiposity syndrome. Diabetol Metab Syndr 8:40. doi:10.1186/s13098-016-0156-2
Jung UJ, Choi MS (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15:6184–6223. doi:10.3390/ijms15046184
Chen L, Chen R, Wang H, Liang F (2015) Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015:508409. doi:10.1155/2015/508409
Kammoun HL, Kraakman MJ, Febbraio MA (2014) Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord 15:31–44. doi:10.1007/s11154-013-9274-4
Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E (2017) The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediators Inflamm 2017:8162421. doi:10.1155/2017/8162421
Nakamura K, Fuster JJ, Walsh K (2014) Adipokines: a link between obesity and cardiovascular disease. J Cardiol 63:250–259. doi:10.1016/j.jjcc.2013.11.006
Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B (2015) The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 45:2446–2456. doi:10.1002/eji.201545502
Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL (2014) Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol 5:470. doi:10.3389/fimmu.2014.00470
Meshkani R, Vakili S (2016) Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta 462:77–89. doi:10.1016/j.cca.2016.08.015
Blüher M (2016) Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci 18:1603–1614. doi:10.1042/CS20160005
Bastard JP, Maachi M, Lagathu C et al (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 1:4–12
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 5091:87–91
Calle MC, Fernandez ML (2012) Inflammation and type 2 diabetes. Diabetes Metab 38:183–191. doi:10.1016/j.diabet.2011.11.006
Mirza S, Hossain M, Mathews C et al (2012) Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 1:136–142. doi:10.1016/j.cyto.2011.09.029
Pickup JC, Chusney GD, Thomas SM, Burt D (2000) Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 67:291–300
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Kim CS, Park HS, Kawada T et al (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes 9:1347–1355
Cavusoglu E, Marmur JD, Yanamadala S et al (2015) Elevated baseline plasma IL-8 levels are an independent predictor of long-term all-cause mortality in patients with acute coronary syndrome. Atherosclerosis 242:589–594. doi:10.1016/j.atherosclerosis.2015.08.022
Ajmera V, Perito ER, Bass NM et al (2017) Novel plasma biomarkers associated with liver disease severity in adults with nonalcoholic fatty liver disease. Hepatology 65:65–77. doi:10.1002/hep.28776
Mukaida N, Shiroo M, Matsushima K (1989) Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol 143(4):1366–1371 PMID: 2663993
Remick DG (2005) Interleukin-8. Crit Care Med 33:S466–S467
Baggiolini M, Loetscher P, Moser B (1995) Interleukin-8 and the chemokine family. Int J Immunopharmacol 17:103–108
Leonard EJ, Yoshimura T (1990) Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am J Respir Cell Mol Biol 2(6):479–486 Review. PMID: 2189453
Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M (1999) Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation 99(3):420–426 PMID: 9918530
Koch AE, Polverini PJ, Kunkel SL et al (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258(5089):1798–1801 PMID: 1281554
Yue TL, Mckenna PJ, Gu JL, Feuerstein GZ (1993) Interleukin-8 is chemotactic for vascular smooth muscle cells. Eur J Pharmacol 240(1):81–84 PMID: 8405125
Gerszten RE, Garcia-Zepeda EA, Lim YC et al (1999) MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398(6729):718–723 PMID: 10227295
Joshi-Barve S, Barve SS, Butt W, Klein J, McClain CJ (2003) Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology 38(5):1178–1187 PubMed PMID: 14578856
Zimmermann HW, Seidler S, Gassler N et al (2011) Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS ONE 6(6):e21381. doi:10.1371/journal.pone.0021381 Epub 2011 Jun 22. PubMed PMID: 21731723; PubMed Central PMCID: PMC3120868
Xu L, Kitade H, Ni Y, Ota T (2015) Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 5(3):1563–1579. doi:10.3390/biom5031563 Review. PubMed PMID: 26197341; PubMed Central PMCID: PMC4598764
Mirza MS (2011) Obesity, visceral fat, and NAFLD: querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterol 2011:592404. doi: 10.5402/2011/592404. Epub 2011 Aug 28. PubMed PMID: 21991518; PubMed Central PMCID: PMC3168494
Kim JS, Lê KA, Mahurkar S, Davis JN, Goran MI (2012) Influence of elevated liver fat on circulating adipocytokines and insulin resistance in obese Hispanic adolescents. Pediatr Obes 7(2):158–164. doi:10.1111/j.2047-6310.2011.00014.x Epub 2012 Feb 9. PubMed PMID: 22434756; PubMed Central PMCID: PMC3767148
Jarrar MH, Baranova A, Collantes R et al (2008) Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 27(5):412–421 Epub 2007 Dec 10 PubMed PMID: 18081738
Chu CJ, Lu RH, Wang SS et al (2007) Plasma levels of interleukin-6 and interleukin-8 in Chinese patients with non-alcoholic fatty liver disease. Hepatogastroenterology 54(79):2045–2048 PubMed PMID: 18251157
Huang YS, Chan CY, Wu JC, Pai CH, Chao Y, Lee SD (1996) Serum levels of interleukin-8 in alcoholic liver disease: relationship with disease stage, biochemical parameters and survival. J Hepatol 24:377–384
Bahcecioglu IH, Yalniz M, Ataseven H et al (2005) Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology 65:1549–1553
Yamaguchi R, Yamamoto T, Sakamoto A et al (2015) Chemokine profiles of human visceral adipocytes from cryopreserved preadipocytes: neutrophil activation and induction of nuclear factor-kappa B repressing factor. Life Sci 143:225–230. doi:10.1016/j.lfs.2015.11.010
Kobashi C, Asamizu S, Ishiki M et al (2009) Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J Inflamm 6:25. doi:10.1186/1476-9255-6-25
Marino F, Tozzi M, Schembri L et al (2015) Production of IL-8, VEGF and elastase by circulating and intraplaque neutrophils in patients with carotid atherosclerosis. PLoS ONE 10:e0124565. doi:10.1371/journal.pone.0124565
Kobashi C, Asamizu S, Ishiki M et al (2009) Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J Inflamm (Lond) 6:25. doi:10.1186/1476-9255-6-25 PubMed PMID: 19709445; PubMed Central PMCID: PMC2746203
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Zozuliñska D, Majchrzak A, Sobieska M, Wiktorowicz K, Wierusz-Wysocka B (1999) Serum interleukin-8 level is increased in diabetic patients. Diabetologia 42:117–118
Esposito K, Nappo F, Giugliano F et al (2003) Cytokine milieu tends toward inflammation in type 2 diabetes. Diabetes Care 26:1647
Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S (2000) Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol 121:437–443
Samaras K, Botelho NK, Chisholm DJ, Lord RV (2010) Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring). 18(5):884–889. doi:10.1038/oby.2009.443
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29(24):2959–2971. doi:10.1093/eurheartj/ehn387
Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L (2014) The obesity paradox in diabetes. Curr Cardiol Rep 16(2):446. doi:10.1007/s11886-013-0446-3
Mancuso P (2016) The role of adipokines in chronic inflammation. Immunotargets Ther 5:47–56. doi:10.2147/ITT.S73223
Kwon H, Pessin JE (2013) Adipokines mediate inflammation and insulin resistance. Front Endocrinol 4:71. doi:10.3389/fendo.2013.00071
Calton EK, Keane KN, Newsholme P, Soares MJ (2015) The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS ONE 10:e0141770. doi:10.1371/journal.pone.0141770
Wei R, Christakos S (2015) Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 7:8251–8260. doi:10.3390/nu7105392
Jung CH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO (2016) Relationship between vitamin D status and vascular complications in patients with type 2 diabetes mellitus. Nutr Res 2:117–124. doi:10.1016/j.nutres.2015.11.008
Li DM, Zhang Y, Li Q, Xu XH, Ding B, Ma JH (2016) Low 25-hydroxy vitamin D level is associated with peripheral arterial disease in type 2 diabetes patients. Arch Med Res 47:49–54. doi:10.1016/j.arcmed.2016.01.007
Hamdy Al-Said N, Abd El Ghaffar Mohamed N, Salam RF, Fawzy MW (2015) Vitamin D as a risk factor for premature atherosclerosis in patients with type 2 diabetes. Ther Adv Endocrinol Metab 6:249–257. doi:10.1177/2042018815600640
Chowdhury R, Kunutsor S, Vitezova A et al (2014) Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomized intervention studies. BMJ 348:g1903. doi:10.1136/bmj.g1903
Funding
This study has been funded by research Grants from the Sapienza University Ateneo Scientific Research (MGC, IB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical standard
All procedures performed in the study were in accordance with the ethical standards of the institutional (Sapienza University of Rome) and national research committee and with the 1964 Helsinki Declaration and its 2008 amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed By Antonio Secchi.
Rights and permissions
About this article
Cite this article
Cimini, F.A., Barchetta, I., Porzia, A. et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol 54, 961–967 (2017). https://doi.org/10.1007/s00592-017-1039-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-1039-1