Abstract
Aims
Previous studies showed that serum 1,5-anhydroglucitol (1,5-AG) levels are significantly reduced in patients with diabetes mellitus (DM). However, it remains unclear how 1,5-AG levels acutely change in response to a glucose load. This study explored acute changes in 1,5-AG levels after a glucose load and the related influencing factors in individuals with differing degrees of glucose tolerance.
Methods
A total of 681 participants (353 without DM and 328 with DM) without a prior history of DM were enrolled. All participants underwent an oral glucose tolerance test. Fasting and postload (30, 60, 120, and 180 min) levels of plasma glucose, serum 1,5-AG, and insulin were measured.
Results
In all participant groups, serum 1,5-AG levels were slightly elevated after a glucose load and reached peak values at 120 min after loading (all P < 0.05). Regression analysis showed that body weight was negatively associated with the difference between peak and baseline 1,5-AG levels (Δ1,5-AG, standardized β = −0.119, P < 0.01). A strong and positive association between 1,5-AG0 and Δ1,5-AG was also found independent of other confounding factors (standardized β = 0.376, P < 0.01). The ratio of the Δ1,5-AG to the 1,5-AG0 was higher in DM patients (7.3% [3.4–11.5%]) than in those without DM (6.2% [3.6–9.2%]).
Conclusions
In contrast to the established decline in 1,5-AG levels with long-term hyperglycemia, the present study showed that serum 1,5-AG levels slightly increased by 6–7% after a glucose load. Further studies in different 1,5-AG transport models are needed to investigate the relevant metabolic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,5-Anhydroglucitol (1,5-AG), a six-carbon chain monosaccharide produced primarily during ingestion, is a 1-deoxy form of glucopyranose with a structure similar to glucose. Importantly, patients with diabetes mellitus (DM) exhibit significantly lower levels of 1,5-AG than normoglycemic individuals. Based on its ability to accurately reflect glucose control over a period of 1–2 weeks [1, 2], 1,5-AG was approved by the US Food and Drug Administration (FDA) in 2003 as a short-term marker of glycemic control [3, 4]. Measurement of the 1,5-AG level is valuable in assessing short-term and postprandial glycemic excursions [5, 6], especially in individuals with a glycated hemoglobin A1c (HbA1c) level less than 8% (64 mmol/mol) [7, 8].
With long-term hyperglycemia, large amounts of urinary glucose are persistently filtered off, which competitively inhibits reabsorption of l,5-AG in the renal proximal tubules [9]. This leads to an increase in the level of excreted l,5-AG and a decrease in the serum 1,5-AG level in patients with DM. Few studies have explored the specific physiological roles of 1,5-AG as well as the corresponding metabolic pathways, specifically in relation to the acute changes in serum 1,5-AG levels after a glucose load, and the results have been inconclusive due to small sample sizes. For example, Akanuma et al. [10] found that 1,5-AG levels remained stable after a glucose load, whereas Goto et al. [11] reported that 1,5-AG levels were significantly increased at 90, 120, and 180 min during an oral glucose tolerance test (OGTT) in 77 healthy Japanese individuals. Therefore, the goal of the present study was to explore the acute changes in serum 1,5-AG levels after glucose loading in individuals with varied degrees of glucose tolerance and to identify the related influencing factors, for the purpose of better understanding the role of 1,5-AG in metabolism and supporting further studies of its transport.
Materials and methods
Study population
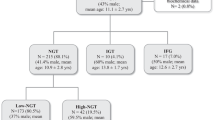
The study population included 681 individuals, including 310 men and 371 women (age range 21–78 years), who were treated in the clinic of the Department of Endocrinology and Metabolism of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital between January 2014 and January 2016. All participants had no history of DM, history of diet control, or current use of hypoglycemic agents. The study population was restricted to those without subtotal gastrectomy, thyroid dysfunction, kidney disease, chronic liver disease, tumors, mental disorders, cystic fibrosis, acute infection, pregnancy, or a history of use of some traditional Chinese medicines such as Polygala Tenuifolia and Senega syrup therapy. This study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Informed consent was provided by all participants prior to enrollment.
Collection of clinical information
Well-trained investigators conducted a questionnaire survey that included questions about participants’ history of past and present illnesses and medication use. Blood pressure, height, and body weight were measured, and body mass index (BMI) was calculated as weight/height2 (kg/m2).
Biochemical measurements
Blood samples from all subjects were obtained in the morning after a 10-h overnight fast to measure the levels of fasting plasma glucose (PG0), fasting serum 1,5-AG (1,5-AG0), fasting serum insulin (INS0), and HbA1c. A 75-g OGTT was administered to each outpatient to assay the postload levels of plasma glucose (PG30, PG60, PG120, and PG180), serum 1,5-AG (1,5-AG30, 1,5-AG60, 1,5-AG120, and 1,5-AG180), and serum insulin (INS30, INS60, INS120, and INS180) at 30, 60, 120, and 180 min, respectively. Plasma glucose levels were immediately obtained by the glucose oxidase method (Kehua Biological Engineering Co., Ltd., Shanghai, China) using the Glamour 2000 biochemical autoanalyzer. Serum 1,5-AG levels were measured by an enzymatic method (GlycoMark™; GlycoMark Inc., New York, NY, USA) on a 7600–120 autoanalyzer (Hitachi, Tokyo, Japan) with inter- and intra-assay coefficients of variation (CV) of 1.54–3.03 and 0.83–2.44%, respectively. HbA1c was detected by high-pressure liquid chromatography (Variant II hemoglobin analyzer; Bio-Rad, Hercules, CA, USA) with inter- and intra-assay CV of 0.75–3.39 and 0.55–2.58%, respectively. Electrochemiluminescence immunoassay was used to quantify the serum insulin levels on a Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) with inter- and intra-assay CV of 2.50 and 1.70%, respectively.
Diagnostic criteria
DM and impaired glucose regulation (IGR) were diagnosed according to the 1999 World Health Organization (WHO) criteria [12]. The 1998 WHO standards were used to classify BMI: participants with BMI ≥25 kg/m2 were classified as overweight/obesity [13].
Calculation of differences in postload 1,5-AG levels
The difference between peak and baseline 1,5-AG levels (Δ1,5-AG) was used to evaluate the range of variation in postload 1,5-AG levels. Then, the ratio of Δ1,5-AG to the baseline 1,5-AG levels (Δ1,5-AG/1,5-AG0) was expressed as a percentage and used to assess the relative differences in postload 1,5-AG levels.
Statistical analysis
SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Based on a normality test, normally distributed data are presented as mean ± standard deviation values, and skewed data are presented as median with interquartile ranges. Pair analyses were carried out using the paired Student’s t test and the Wilcoxon signed-rank sum test. Intergroup comparisons were conducted with the unpaired Student’s t test, Kruskal–Wallis test, and Chi-squared test for continuous data with a normal distribution, continuous data with a skewed distribution, and categorical variables, respectively. Spearman correlation analysis and multiple stepwise regression analysis were conducted to identify independent factors influencing Δ1,5-AG levels. A two-tailed P value < 0.05 was considered to be statistically significant.
Results
Clinical characteristics of outpatient study participants
The average age of the 681 study participants was 53 years (39–61 years), and the average HbA1c level was 5.9% (5.5–6.5%) (41 mmol/mol [37–48 mmol/mol]). Among the entire study population, 184 participants exhibited normal glucose tolerance (NGT, 27.0%, 59 men and 125 women), 169 presented with IGR (24.8%, 75 men and 94 women), and 328 were diagnosed with DM (48.2%, 176 men and 152 women). Compared with the NGT group, the DM and IGR groups showed higher values of age, systolic blood pressure, body weight, BMI, HbA1c, PG0, PG30, PG60, PG120, and PG180 (all P < 0.05), along with lower levels of INS0, INS30, INS120, and INS180 (all P < 0.05; Table 1).
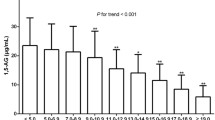
The l,5-AG0, l,5-AG30, l,5-AG60, l,5-AG120, and l,5-AG180 levels were significantly lower in the DM group than in the NGT and IGR groups (P < 0.01). However, the serum 1,5-AG levels did not differ significantly between the NGT and IGR groups (P > 0.05).
Acute changes in serum 1,5-AG levels after OGTT
Postload 1,5-AG levels at each time point were slightly higher than baseline levels and reached peak values at 120 min in all three groups (all P < 0.01; Fig. 1). The same results were obtained in separate analyses for men and women as well as for overweight/obesity and non-overweight/non-obesity participants.
The mean Δ1,5-AG values were 1.4 μg/mL (0.7–2.3 μg/mL), 1.4 μg/mL (0.7–2.2 μg/mL), and 0.9 μg/mL (0.5–1.4 μg/mL) for the NGT, IGR, and DM groups, respectively. These results demonstrated that the Δ1,5-AG was significantly lower in patients with DM than in individuals with IGR or NGT (both P < 0.01), whereas the Δ1,5-AG did not differ significantly between those with IGR and NGT (P = 0.400).
Factors independently associated with Δ1,5-AG
Spearman correlation analysis revealed that the Δ1,5-AG was negatively associated with body weight, BMI, HbA1c, INS0, PG0, PG30, PG60, PG120, and PG180 (all P < 0.05). Further multiple stepwise regression models were performed to identify factors independently affecting the range of variation in postload 1,5-AG levels, with Δ1,5-AG defined as the dependent variable. Model 1 included gender, age, BMI, HbA1c, PG0, PG30, PG60, PG120, PG180, and INS0 as independent variables and showed that Δ1,5-AG was independently related to PG120, BMI, and age (standardized β = −0.261, −0.109, and 0.131, respectively; all P < 0.01). In Model 2, body weight was included as independent variable to replace BMI, and the results indicated that Δ1,5-AG was significantly associated with PG120, body weight, and age (standardized β = −0.254, −0.136, and 0.121, respectively; all P < 0.01). A comparison of the standardized β values for BMI and body weight revealed that body weight had a more significant negative impact on Δ1,5-AG. Postload 1,5-AG excursions were inversely associated with body weight.
When the 1,5-AG0 level was added to Model 3, the results showed that in addition to body weight (standardized β = –0.119, P < 0.01), 1,5-AG0 positively influenced Δ1,5-AG (standardized β = 0.376, P < 0.01; Table 2). Furthermore, when Model 3 was performed separately for participants with and without DM, the results showed 1,5-AG0, body weight, and age all contributed to Δ1,5-AG in individuals without DM (standardized β = 0.372, −0.109, and 0.105, respectively; all P < 0.05; Fig. 2), and that 1,5-AG0, body weight, and PG120 contributed to Δ1,5-AG in patients with DM (standardized β = 0.189, −0.142, and −0.148, respectively; all P < 0.05; Fig. 2).
Effect of baseline serum 1,5-AG levels on Δ1,5-AG
As the regression analysis models showed, the baseline 1,5-AG level was the main factor independently related to postload 1,5-AG excursions. The Δ1,5-AG/1,5-AG0 was 6.6% (3.5–10.1%) among all participants and higher in DM patients than in participants without DM (7.3% [3.4–11.5%] vs. 6.2% [3.6–9.2%], P < 0.05). The Δ1,5-AG/1,5-AG0 differed significantly neither between men and women (6.6% [3.4–10.7%] vs. 6.5% [3.6–9.5%]) nor between overweight/obesity and non-overweight/non-obesity participants (6.5% [3.5–10.7%] vs. 6.6% [3.4–9.8%]).
Discussion
The current study provides the first analysis of acute changes in serum 1,5-AG levels after a glucose load and the related influencing factors in a Chinese population. The results showed that serum 1,5-AG levels were slightly elevated after a glucose load rather than reduced. These results held true for individuals with different glucose metabolism statuses. Regression analysis showed that in addition to the independent negative association with body weight, the baseline 1,5-AG level was an independent factor more strongly contributing to a larger range of variation in the postload 1,5-AG levels. The 1,5-AG levels increased by 6–7% from the baseline 1,5-AG levels, and no association was found between insulin levels and the Δ1,5-AG.
In addition to research demonstrating that 1,5-AG can accurately and rapidly reflect glucose control over a period of 1–2 weeks [14, 15], previous studies have suggested that 1,5-AG is superior to glycated albumin (GA) and HbA1c for the evaluation of postprandial glucose variability. Sun et al. [16] found that 1,5-AG, rather than HbA1c and GA, is significantly correlated with postprandial glucose excursions and the postprandial incremental area under the curve in moderately and well-controlled patients. Pramodkumar et al. [17] measured fasting serum levels of 1,5-AG, fructosamine, and HbA1c in Asian Indians with different degrees of glucose intolerance. Their results showed that circulating levels of 1,5-AG became progressively lower as glucose intolerance increased, with individuals with NGT having the highest levels followed by those with IGR and DM, which is consistent with the findings in the present study. The serum 1,5-AG levels also have been suggested to be closely associated with diabetic retinopathy and cardiovascular diseases [18,19,20]. Thus, the 1,5-AG levels may be useful for identifying individuals at higher risk of these conditions [21,22,23,24,25]. Furthermore, in vitro studies have shown that 1,5-AG may regulate hepatic glucose metabolism by affecting both the glycogenolysis and gluconeogenesis pathways [26] and play an important role in the regulation of glucose-mediated insulin secretion [27].
Notably, we found that the serum 1,5-AG levels increased slightly, instead of declining, with the increase in blood glucose levels after a glucose load test. To date, few studies have evaluated the acute changes in 1,5-AG levels after glucose loading. The study reported by Akanuma et al. [10] included 5 participants with NGT, 7 with IGR, and 5 with DM who underwent a 100-g OGTT. They observed that the 1,5-AG levels remained stable after an acute glucose load. In addition, a study [11] involving 77 healthy Japanese men found no significant changes in 1,5-AG levels between baseline and either 30 min or 60 min after a 75-g glucose load, but they observed significant increases in 1,5-AG levels at 90, 120, and 180 min after glucose loading, which is somewhat consistent with the findings in the present study.
The mechanisms underlying the minor changes in 1,5-AG levels after an acute glucose load are unknown. Previous studies revealed a kinetic mass balance model for 1,5-AG among kidney, plasma, and tissue pools [28]. Studies also showed that a drastic efflux of 1,5-AG from all tissues into the blood can be induced by the acute hyperglycemia [29, 30]. Therefore, we speculated that with the transport of a large amount of glucose into cells upon glucose loading, 1,5-AG would move from the intracellular space to the extracellular space and thereby cause the increase in circulating 1,5-AG levels after an acute glucose load. Moreover, our study indicated that postload 1,5-AG excursions were predominately and independently related to the baseline 1,5-AG level, which might be explained by the scale of the 1,5-AG pool in the entire body. Further research is needed to determine whether a shared membrane transport system is responsible for the transport of 1,5-AG and glucose.
Notably, we also found that body weight was a negative independent factor affecting postload 1,5-AG excursions. The underlying mechanisms might relate to the volume of body fluid, which can be reflected by body weight. With equal amounts of transcellular 1,5-AG evenly distributed in the extracellular fluid, a greater volume of body fluid might correspond to a decrease in Δ1,5-AG.
Because baseline 1,5-AG levels differed between individuals with and without DM, we further analyzed factors influencing postload 1,5-AG excursions in these two populations. Our results showed that in addition to body weight and 1,5-AG0 levels, PG120 was a positive independent factor for postload 1,5-AG excursions only in DM patients, and age was a negative independent factor for postload 1,5-AG excursions only in those without DM. The PG120 can reflect circulating glucose concentrations, and an increasing trend could indicate a decreasing trend in the amount of glucose transported into cells under an equivalent glucose load (75 g) and a corresponding downtrend in the amount of 1,5-AG transported out of cells. In contrast, in individuals without DM, because of the relatively narrow range of postprandial blood glucose fluctuations, the impact of PG120 on Δ1,5-AG was not obvious. The mechanism responsible for the effect of age on the Δ1,5-AG in participants without DM is not clear. Age might affect the Δ1,5-AG via a decline in the stability of the cell membrane, which could mean that more time is required to restore the balance of intracellular and extracellular 1,5-AG levels.
The present study indicated the serum 1,5-AG levels were slightly elevated by 6–7% rather than reduced after a glucose load, and no differences were found among participants of different gender, glucose metabolism status, or BMI, which suggests that it is reasonable to choose non-fasting 1,5-AG measurements for clinical use.
There are some limitations to this study. First, glycemic variability was only evaluated within a period of 180 min after administration of a 75-g glucose load. A longer observation period would provide a more complete picture of the changes in serum 1,5-AG levels. Second, the present study explored acute changes in 1,5-AG levels after only a 75-g glucose load. Moreover, further studies are needed to explore changes in 1,5-AG levels after an acute glucose load in individuals on different diets.
In conclusion, the present study demonstrated that, unlike the change associated with long-term hyperglycemia, the serum 1,5-AG levels were slightly elevated by 6–7% rather than reduced after a glucose load, and the results provided the first evidence that the baseline 1,5-AG level and body weight were independent and positive factors associated with the variation of postload 1,5-AG levels. Because the mechanism for this change remains unclear, further studies in different 1,5-AG transport models are needed to investigate the relevant metabolic pathways.
References
Koga M (2014) 1,5-Anhydroglucitol and glycated albumin in glycemia. Adv Clin Chem 64:269–301
Ma X, Hao Y, Hu X et al (2015) 1,5-anhydroglucitol is associated with early-phase insulin secretion in chinese patients with newly diagnosed type 2 diabetes mellitus. Diabetes Technol Ther 17(5):320–326
McGill JB, Cole TG, Nowatzke W et al (2004) Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care 27(8):1859–1865
Nerby CL, Stickle DF (2009) 1,5-anhydroglucitol monitoring in diabetes: a mass balance perspective. Clin Biochem 42(3):158–167
Stettler C, Stahl M, Allemann S et al (2008) Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care 31(8):1534–1535
Dungan KM, Buse JB, Largay J et al (2006) 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 29(6):1214–1219
Kim MJ, Jung HS, Hwang-Bo Y et al (2013) Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol 50(4):505–510
Kim WJ, Park CY (2013) 1,5-Anhydroglucitol in diabetes mellitus. Endocrine 43(1):33–40
Yamanouchi T, Shinohara T, Ogata N, Tachibana Y, Akaoka I, Miyashita H (1996) Common reabsorption system of 1,5-anhydro-d-glucitol, fructose, and mannose in rat renal tubule. Biochim Biophys Acta 1291(1):89–95
Akanuma Y, Morita M, Fukuzawa N, Yamanouchi T, Akanuma H (1988) Urinary excretion of 1,5-anhydro-d-glucitol accompanying glucose excretion in diabetic patients. Diabetologia 31(11):831–835
Goto M, Yamamoto-Honda R, Shimbo T et al (2011) Correlation between baseline serum 1,5-anhydroglucitol levels and 2-h post-challenge glucose levels during oral glucose tolerance tests. Endocr J 58(1):13–17
Department of Noncommunicable Disease Surveillance (1999) Report of a WHO Consultation: definition, diagnosis and classification of diabetes mellitus and its complication: Part1: diagnosis and classification of diabetes mellitus. World Health Organization, Geneva
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894:1–253
Nowak N, Skupien J, Cyganek K, Matejko B, Malecki MT (2013) 1,5-Anhydroglucitol as a marker of maternal glycaemic control and predictor of neonatal birthweight in pregnancies complicated by type 1 diabetes mellitus. Diabetologia 56(4):709–713
Wang Y, Yuan Y, Zhang Y et al (2016) Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol Nov 28 [Epub ahead of print]
Sun J, Dou JT, Wang XL et al (2011) Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. Chin Med J (Engl) 124(22):3641–3645
Pramodkumar TA, Jayashri R, Gokulakrishnan K et al (2016) Relationship of glycemic control markers—1,5 anhydroglucitol, fructosamine, and glycated hemoglobin among Asian Indians with different degrees of glucose intolerance. Indian J Endocrinol Metab 20(5):690–695
Ma X, Hu X, Zhou J et al (2015) Glycated albumin is more closely correlated with coronary artery disease than 1,5-anhydroglucitol and glycated hemoglobin A1c. Cardiovasc Diabetol 14:16
Selvin E, Rawlings A, Lutsey P et al (2016) Association of 1,5-Anhydroglucitol with cardiovascular disease and mortality. Diabetes 65(1):201–208
Kim WJ, Park CY, Park SE et al (2012) Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet Med 29(9):1184–1190
Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T (2011) Serum 1,5-anhydro-d-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis 216(2):477–483
Ikeda N, Hara H, Hiroi Y (2015) Ability of 1,5-anhydro-d-glucitol values to predict coronary artery disease in a non-diabetic population. Int Heart J 56(6):587–591
Ikeda N, Hara H, Hiroi Y (2014) 1,5-Anhydro-d-glucitol predicts coronary artery disease prevalence and complexity. J Cardiol 64(4):297–301
Fujiwara T, Yoshida M, Yamada H et al (2015) Lower 1,5-anhydroglucitol is associated with denovo coronary artery disease in patients at high cardiovascular risk. Heart Vessels 30(4):469–476
Takahashi S, Shimada K, Miyauchi K et al (2016) Low and exacerbated levels of 1,5-anhydroglucitol are associated with cardiovascular events in patients after first-time elective percutaneous coronary intervention. Cardiovasc Diabetol 15(1):145
Kato A, Kunimatsu T, Yamashita Y, Adachi I, Takeshita K, Ishikawa F (2013) Protective effects of dietary 1,5-anhydro-d-glucitol as a blood glucose regulator in diabetes and metabolic syndrome. J Agric Food Chem 61(3):611–617
Yamanouchi T, Inoue T, Ichiyanagi K, Sakai T, Ogata N (2003) 1,5-Anhydroglucitol stimulates insulin release in insulinoma cell lines. Biochim Biophys Acta 1623(2–3):82–87
Stickle D, Turk J (1997) A kinetic mass balance model for 1,5-anhydroglucitol: applications to monitoring of glycemic control. Am J Physiol 273(4 Pt 1):E821–E830
Yamanouchi T, Akaoka I, Akanuma Y, Akanuma H, Miyashita E (1990) Mechanism for acute reduction of 1,5-anhydroglucitol in rats treated with diabetogenic agents. Am J Physiol 258(3 Pt 1):E423–E427
Yamanouchi T, Akanuma H, Takaku F, Akanuma Y (1986) Marked depletion of plasma 1,5-anhydroglucitol, a major polyol, in streptozocin-induced diabetes in rats and the effect of insulin treatment. Diabetes 35(2):204–209
Acknowledgements
This work was funded by the Shanghai Municipal Science and Technology Commission Medical Guide Project (15411963500), the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China (2016D01C084), and the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430).
Author contribution
J.Z. and W.J. designed the study. X.M., H.S., and X.H. collected data. H.S. analyzed data and wrote the draft. X.M., J.Y., and Y.W. provided technical support. J.Z., Y.B., and W.J. revised the paper and contributed to the discussion. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Ethical disclosure
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Federici.
Hang Su, Xiaojing Ma and Jun Yin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Su, H., Ma, X., Yin, J. et al. Serum 1,5-anhydroglucitol levels slightly increase rather than decrease after a glucose load in subjects with different glucose tolerance status. Acta Diabetol 54, 463–470 (2017). https://doi.org/10.1007/s00592-017-0968-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0968-z