Abstract
Aims
We investigated associations between serum levels of glycated albumin (GA) and glycated hemoglobin (HbA1c) and the presence of diabetic peripheral neuropathy (DPN) in patients with type 1 diabetes mellitus (T1DM).
Methods
Between September 2009 and April 2015, we evaluated 314 patients with T1DM in the Endocrinology Department of Shengjing Hospital of China Medical University. We divided the patients into the DPN group (n = 72) and the non-DPN group (n = 242), on the basis of the presence of DPN.
Results
The DPN group had significantly higher GA values than the non-DPN group. After univariate logistic regression, we selected several factors for further analysis: HbA1c, GA, duration of T1DM, body mass index, smoking, hypertension, and the presence of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease. We performed a multivariate logistic regression analysis to examine the association between the presence of DPN and each of these variables. We identified GA, HbA1c, hypertension, smoking, retinopathy, and cardiovascular disease as independent variables for indicating the presence of DPN. Results of a receiver operating characteristic curve analysis revealed that the area under the curve of GA (0.771) was larger than that of HbA1c (0.629). We defined the cutoff value of GA as 23.5 % (sensitivity 0.764, specificity 0.661) and the cutoff value of HbA1c as 8.45 % (sensitivity 0.667, specificity 0.595) for predicting DPN in patients with T1DM.
Conclusions
GA may be a better indicative marker of DPN in patients with T1DM than HbA1c.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a prevalent disease worldwide, and it currently affects more than 387 million people globally. By 2035, DM is expected to affect approximately 592 million people [1]. An estimated 60–70 % of patients with DM suffer from neuropathies [2], and diabetic peripheral neuropathy (DPN) is the most common form of neuropathy among diabetic patients. DPN can lead to poor physical function, loss of postural control, and impaired balance. These changes affect activities of daily living and increase the risk of falling, which can lead to morbidity and decreased quality of life [3]. DPN also contributes to the worsening of ischemic symptoms and foot ulcerations in patients with peripheral arterial occlusive disease [4]. Therefore, DPN is considered to be a primary cause of morbidity and mortality among patients with DM [5]. The mechanisms for the development of neuropathies in DM remain unclear, but they are likely multifactorial and involve environmental and lifestyle factors, as well as genetic predispositions [6].
Glucose control is the only identified mean for effectively preventing DPN and, as such, management of blood glucose is important for patients with DM. Glycated hemoglobin (HbA1c) is the gold standard for assessing glycemic control, and research has shown that HbA1c is a strong independent predictor of diabetic neuropathy. However, the use of HbA1c has a significant limitation: It only quantifies long-term glucose control over a 2–3-month period.
Glycated albumin (GA) measures the amount of albumin that is bound to glucose as a percentage of total serum albumin. The measurement of GA is not affected by the serum albumin level. GA is a marker of short-term (2–4 weeks) glycemic control. So, it complements information obtained from HbA1c measurements for identifying patients at risk for developing DM and complications of DM, especially in angiopathies [4, 7–9]. In fact, GA may be superior to HbA1c as a predictor of DPN. We investigated the associations between serum levels of GA and HbA1c and the presence of DPN in patients with type 1 DM (T1DM). We also aimed to determine whether GA is more useful than HbA1c in predicting DPN.
Materials and methods
Experiment design
We selected 314 patients with T1DM between September 2009 and April 2015 from the Endocrinology Department of Shengjing Hospital of China Medical University. We divided the patients on the basis of the presence of DPN into the DPN group and the non-DPN group. We collected and recorded general medical conditions, biochemical profiles, DPN-related indexes, medical histories, and HbA1c and GA levels for each patient. We analyzed each of these factors by univariate logistic regression analysis to select variables associated with the presence of DPN. We selected related variables for further analysis by multivariate logistic regression analysis. Finally, we performed a receiver operating characteristic (ROC) curve analysis to compare the utility of GA and HbA1c as indicators of the presence of DPN. The protocol for this study was approved by the ethics committee of Shengjing Hospital of China Medical University, and all patients provided written informed consent for participation.
We excluded patients with infectious diseases, inflammatory diseases, liver failure, malignancies, history of neurodegenerative diseases, history of cerebrovascular diseases, history of diseases in the cervical or lumbar spine, history of serious trauma to the limbs, use of neurotoxic medication within the last 3 months, vitamin B12 deficiency, and excessive alcohol consumption.
DPN diagnosis
The diagnosis of diabetic peripheral neuropathy was made according to clinical symptoms, neurologic examination, and electrophysiologic investigation. Nerve conduction studies were performed with standard electromyography equipment.
Symptoms of DPN included decreased sensation, positive neuropathic sensory symptoms (“asleep numbness”, prickling or stabbing, burning or aching pain, etc.) predominantly in the toes, feet, or legs. Signs included symmetric decrease in distal sensation or unequivocally decreased or absent ankle reflexes. Touch sensation was tested with a 10-g monofilament on four sites per foot, pain sensation was revealed with a pin, reflexes were examined with a tendon hammer, and vibration sensation was tested with a standard 128-Hz tuning fork [10, 11].
The motor nerve conduction velocity (MCV) and the sensory nerve conduction velocity (SCV) of each patient were measured by EMG (EMG/EP 6200A Systems, Cadwell Laboratories Inc., Kennewick, WA, USA). Generally speaking, stimuli were added at both the distal end and the proximal end. The MCV and SCV values were the quotient of the distance between the two stimulation points divided by the latency difference between the two points.
Measurement of GA
GA was measured using an enzymatic method (Lucica GA-L protocol, Asahi Kasei Corp., Beijing, China). Generally speaking, this assay included separated measurement of total albumin and glycated albumin. Glycated albumin measurement utilized ketoamine oxidase and an albumin-specific protease. The result was expressed as the percentage of serum glycated albumin to total albumin.
Assessment of general condition
We evaluated several risk factors for DPN in all patients: age, sex, body mass index (BMI), duration of DM, levels of GA and HbA1c, insulin dose, systolic blood pressure (SBP), diastolic blood pressure (DBP), urinary albumin-to-creatinine ratio(UACR), levels of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C),smoking status, the presence of retinopathy, and cardiovascular disease.
BMI was calculated as weight (kg)/height (m)2. Blood pressure was determined as the mean of two measurements obtained in an office setting by a conventional cuff method using a mercury sphygmomanometer after at least 5 min of rest. Patients with an SBP ≥140 mmHg and/or a DBP ≥90 mmHg or who were receiving antihypertensive drugs were considered to have hypertension. Information on the duration of DM and medication use was obtained from medical records.
Assessment of diabetic complications
Diabetic nephropathy (DN) status was determined on the basis of questionnaires, clinical features, and laboratory data. The urinary albumin-to-creatinine ratio (UACR) >300 mg/g, or UACR between 30 and 300 mg/g in the presence of diabetic retinopathy, or microalbuminuria with at least 10-year duration was used to diagnose DN [12].
The presence and severity of diabetic retinopathy were assessed from retinal photographs (two fields per eye) obtained with a wide-angle camera and scored centrally by an expert in evaluating changes in retinopathy.
Cardiovascular disease was defined as either a history of physician-diagnosed cardiovascular disease (e.g., previous myocardial infarction, angina, coronary artery bypass grafting, or stroke) or ischemic changes detected on a 12-lead electrocardiogram [13].
Patients were considered to have dyslipidemia if they were receiving lipid-lowering therapy or if they met the following criteria: LDL-C ≥ 2.6 mmol/L for patients with coronary heart disease or ≥1.8 mmol/L for patients without coronary heart disease; TG ≥ 1.7 mmol/L; and/or HDL-C < 1.0 mmol/L for men or <1.3 mmol/L for women [14].
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as the mean ± standard deviation. Categorical and continuous variables were compared between the groups by Chi-square analysis and independent t test, respectively. When continuous variables did not show a normal distribution, a Wilcoxon rank-sum test was performed. A multivariable logistic regression analysis was used for the multivariate analysis to evaluate independent indicators of DPN. An ROC curve analysis was used to determine the cutoff values for GA and HbA1c. A value of P < 0.05 was considered statistically significant.
Results
General patient characteristics
Patients in the DPN group were older, had a higher BMI, had a longer duration of DM, and had higher levels of HbA1c, GA, UACR, and blood lipids than patients in the non-DPN group. Patients in the DPN group also had higher incidences of diabetic nephropathy, retinopathy, and cardiovascular disease (Table 1).
We analyzed all variables of DPN using univariate logistic regression analysis. DPN was independently associated with HbA1c [odds ratio (OR) = 1.54, 95 % confidence interval (CI) = 1.14–1.85; P < 0.001] and GA (OR = 1.63, 95 % CI = 1.23–1.91; P < 0.001). DPN was also associated with duration of DM (OR = 1.76, 95 % CI = 1.23–1.96; P = 0.013), smoking (OR = 1.43, 95 % CI = 1.05–1.74; P < 0.001), hypertension (OR = 1.78, 95 % CI = 1.25–2.53; P < 0.001), dyslipidemia (OR = 1.28, 95 % CI = 1.04–1.39; P = 0.03), and the presence of diabetic complications, including nephropathy (OR = 1.65, 95 % CI = 1.08–2.14; P = 0.02), retinopathy (OR = 1.48, 95 % CI = 1.05–2.12; P = 0.004), and cardiovascular disease (OR = 1.84, 95 % CI = 1.49–3.15, P < 0.001) (Fig. 1).These associated factors were further analyzed by advanced multivariable logistic regression.
Variables of diabetic peripheral neuropathy (DPN) in patients with type 1 diabetes mellitus (T1DM) according to univariate logistic regression analysis. DPN was independently associated with levels of glycated hemoglobin(HbA1c) and glycated albumin (GA), as well as duration of DM, body mass index (BMI), smoking, hypertension, dyslipidemia, and the presence of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease
Variables of DPN by multivariable logistic regressions
On the basis of the results of the univariate logistic regression analysis, we chose HbA1c, GA, duration of DM, smoking, hypertension, dyslipidemia, and the presence of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease, as indicators to be further evaluated by multivariable logistic regression analysis. In the multiregression, we adjusted possible confounding by the two major known variables related with DPN, age and duration. After adjustment, the variables that remained significantly associated with the presence of neuropathy were GA (OR = 1.48, 95 % CI = 1.11–1.73; P < 0.001), HbA1c (OR = 1.39, 95 % CI = 1.12–1.60; P < 0.001), hypertension (OR = 1.42, 95 % CI = 1.05–1.93; P < 0.001), smoking (OR = 1.37, 95 % CI = 1.08–1.74; P < 0.001), retinopathy (OR = 1.85, 95 % CI = 1.35–2.01; P < 0.001), and cardiovascular disease(OR = 1.64, 95 % CI = 1.10–2.92; P < 0.001). (Table 2; Fig. 2).
Indicators of diabetic peripheral neuropathy (DPN) in patients with type 1 diabetes mellitus (T1DM) according to multivariable logistic regression analysis.Glycated albumin (GA), glycated hemoglobin (HbA1c), duration of DM, body mass index (BMI), hypertension, smoking, and the presence of cardiovascular disease were independent variables of DPN
Cutoff values for GA and HbA1c
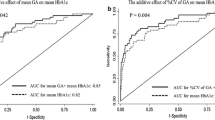
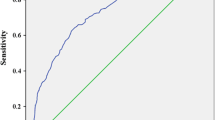
We performed an ROC curve analysis to calculate the areas under the curves (AUC) of GA and HbA1c for the presence of DPN. GA had a larger AUC than HbA1c (AUC = 0.771, 95 % CI = 0.711–0.832; P < 0.000 and AUC = 0.629, 95 % CI = 0.557–0.701; P = 0.001, respectively). The cutoff values of GA and HbA1c that provided the highest sensitivity and specificity for the diagnosis of DPN in patients with T1DM were 23.5 % (sensitivity = 0.764, specificity = 0.661) and 8.45 % (sensitivity = 0.667, specificity = 0.595), respectively (Fig. 3).
Discussions
In this study, we aimed to detect whether GA is a better and a more clinically significant parameter than HbA1c in predicting the risk of DPN. We found that GA, HbA1c, hypertension, smoking, retinopathy, and cardiovascular disease were independent indicators of the presence of DPN in all patients. We also concluded that GA may be superior to HbA1c as a marker for evaluating the presence of DPN in patients with T1DM.
The half-life of serum albumin is shorter than that of erythrocytes. Therefore, GA represents glycemic control over a short period of 2–3 weeks; in comparison, HbA1c represents glycemic control over a period of 8–12 weeks [15–18]. Research has shown that GA is superior to HbA1c in monitoring glucose control [19] and GA is more closely related to glycemic fluctuations than HbA1c in diabetic patients with poor glycemic control who use a continuous glucose monitoring system [20].
However, the linkage to average glucose and their prognostic significance of glycated albumin is not as clear as for A1C [21], and some recent research on GA has shown its relationship with diabetic complications. Selvin et al. reported that GA is a more useful predictor for the presence of microangiopathy, including nephropathy and retinopathy, than HbA1c [22]. Research has also shown that HbA1c is a strong predictor of diabetic neuropathy [23–26], but, until now, there has been no investigation of the relationship between GA and diabetic neuropathy. Accumulating data indicate that GA provides a better representation of glycemic fluctuation, so GA may be a better index for predicting DPN. Here, we observed that, in fact, GA was a better indicator of DPN than HbA1c. Our finding suggests that GA, as an assessment of glycemic control, is more useful for indicating the presence of DPN than HbA1c.
Previous studies reported that neuropathy is associated with other diabetic complications, such as diabetic nephropathy and proliferative retinopathy [27, 28]. But, others suggested that these complications are not necessarily related to diabetic neuropathy. We included glycosation indicators, biochemical indexes, and disease-related complications as part of the analysis in this study, and our findings suggest that diabetic nephropathy is not independent variables for DPN. This agrees with the findings of Tesfaye et al. [13] who found that the relationship between nephropathy and DPN was insignificant when changes in the HbA1c value were included in model.
From our study, we found GA had a better ability than HbA1c to discriminate between individuals with and without DPN. This gives us some enlightenment that GA could be used in clinic for the evaluation of glucose control and DPN, although its clinical relevance is not completely clear yet. It can reflect recent glucose fluctuations and complications better than HbA1c. So, it can add data in clinical care and used as a complementary index of HbA1c.
Our study has several limitations. First, we only included 314 patients from a single hospital. Second, we did not have follow-up data for patients, so we were not able to observe the effects of GA on the progression of DPN. However, measurement of GA began only recently in a few hospitals in China, so GA data are difficult to collect. This is the first study to evaluate the presence of DPN according to glycosation indicators in Asian patients with T1DM. Further large-scale, prospective studies are needed to determine whether GA is a suitable indicator for predicting DPN.
In conclusion, we investigated the association between glycemic-related variables and the presence of DPN in patients with T1DM. Our findings suggest that GA is an independent variable for the presence of DPN in patients with T1DM and that the indicative value of GA is stronger than that of HbA1c.
References
International Diabetes Federation (2014) IDF diabetes atlas [Internet]. 6th edn. http://www.idf.org/diabetesatlas
Witzel II, Jelinek HF, Khalaf K, Lee S, Khandoker AH, Alsafar H (2015) Identifying common genetic risk factors of diabetic neuropathies. Front Endocrinol (Lausanne) 6:88. doi:10.3389/fendo.2015.00088
Botelhoa MC, Conde MG, Rebelo Braz NM (2015) Functional aspects in ageing adults with diabetic neuropathy. A review. Curr Diabetes Rev 12(2):114–119. doi:10.2174/1573399811666150722125612
Kim YA, Kim ES, Hwang HK et al (2014) prevalence and risk factors for the peripheral neuropathy in patients with peripheral arterial occlusive disease. Vasc Spec Int 30(4):125–132. doi:10.5758/vsi.2014.30.4.125
Spallone V, Ziegler D, Freeman R et al (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 27(7):639–653. doi:10.1002/dmrr.1239
Wilson NM, Wright DE (2012) Inflammatory mediators in diabetic neuropathy. J Diabetes Metab s5(4):1–6. doi:10.4172/2155-6156.S5-004
Huh JH, Kim KJ, Lee BW et al (2014) The relationship between BMI and glycated albumin to glycated hemoglobin (GA/A1c) ratio according to glucose tolerance status. PLoS One 9(2):e89478. doi:10.1371/journal.pone.0089478
Hwang YC, Jung CH, Ahn HY et al (2014) Optimal glycated albumin cutoff value to diagnose diabetes in Korean adults: a retrospective study based on the oral glucose tolerance test. Clin Chim Acta 437:1–5. doi:10.1016/j.cca.2014.06.027
Kim KJ, Lee BW (2012) The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J 36(2):98–107. doi:10.4093/dmj.2012.36.2.98
Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC (2012) Serum glycated albumin predicts the progression of carotid arterial atherosclerosis. Atherosclerosis 225(2):450–455. doi:10.1016/j.atherosclerosis.2012.09.005
Xu F, Zhao LH, Su JB et al (2014) The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabeto Metab Syndr 6(1):139. doi:10.1186/1758-5996-6-139
Levin A, Rocco M (2007) KDOQI Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49(2):S10–S179
Tesfaye S, Boulton AJ, Dyck PJ et al (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10):2285–2293. doi:10.2337/dc10-1303
Tesfaye S, Chaturvedi N, Eaton SE et al (2005) Vascular risk factors and diabetic neuropathy. N Engl J Med 352(4):341–350. doi:10.1056/NEJMoa032782
Diabetes branch of Chinese Medical Association (2014) Guideline for T2DM in China, 2013 Edition. Chin J Endocrinol Metab 30(10):893–942
Tahara Y, Shima K (1995) Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 18(4):440–447. doi:10.2337/diacare.18.4.440
Takahashi S, Uchino H, Shimizu T et al (2007) Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J 54(1):139–144. doi:10.1507/endocrj.K06-103
Ai M, Otokozawa S, Schaefer EJ et al (2009) Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin Chim Acta 406(1–2):71–74. doi:10.1016/j.cca.2009.05.015
Koga M, Hashimoto K, Murai J et al (2011) Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin Chim Acta 412(3–4):253–257. doi:10.1016/j.cca.2010.10.014
Yoshiuchi K, Matsuhisa M, Katakami N et al (2008) Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 55(3):503–507. doi:10.1507/endocrj.K07E-089
American Diabetes Association (2016) Standards of medical care in diabetes-2016. Diabetes Care 39(s1):s39–s46
Suwa T, Ohta A, Matsui T et al (2010) Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J 57(2):135–140. doi:10.1507/endocrj.K09E-234
Selvin E, Francis LM, Ballantyne CM et al (2011) Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 34(4):960–967. doi:10.2337/dc10-1945
Tesfaye S, Stevens LK, Stephenson JM et al (1996) Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 39(11):1377–1384. doi:10.1007/s001250050586
Maser RE, Steenkiste AR, Dorman JS et al (1989) Epidemiological correlates of diabetic neuropathy. Report from pittsburgh epidemiology of diabetes complications study. Diabetes 38(11):1456–1461. doi:10.2337/diabetes.38.11.1456
Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF (1990) Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley diabetes study. Am J Epidemiol 131(4):633–643
Dyrberg T, Benn J, Christiansen JS, Hilsted J, Nerup J (1981) Prevalence of diabetic autonomic neuropathy measured by simple bedside tests. Diabetologia 20(3):190–194
Hreidarsson AB (1982) Pupil size in insulin-dependent diabetes: relationship to duration, metabolic control, and long-term manifestations. Diabetes 31(5):442–448. doi:10.2337/diabetes.31.5.442
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81301627 and 81500628); Liaoning Province Doctor Startup Foundation (No. 20131146).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Human and animal rights
All procedures followed were in accordance with the ethical standards of ethics committee of Shengjing Hospital of China Medical University on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Wang, N., Guo, C., Han, P. et al. Glycated albumin indicates peripheral diabetic neuropathy. Acta Diabetol 53, 973–979 (2016). https://doi.org/10.1007/s00592-016-0900-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0900-y