Abstract
Aims
To evaluate the effect of cumulative smoking on the development of diabetic nephropathy.
Methods
Study included 3613 patients with type 1 diabetes, participating in the Finnish Diabetic Nephropathy Study. The 12-year cumulative risk of microalbuminuria, macroalbuminuria and end-stage renal disease (ESRD) was estimated for current, ex- and nonsmokers. Cox regression analyses, with multivariable adjustments for other risk factors for diabetic nephropathy, were used to evaluate the risk at different stages of diabetic nephropathy based on the cumulative amount of smoking in pack-years.
Results
The 12-year cumulative risk of microalbuminuria was 18.9 % (95 % CI 14.6–23.0, P < 0.0001) for current smokers and 15.1 % (10.3–19.6, P = 0.087) for ex-smokers, compared with 10.0 % (7.8–12.1) for nonsmokers. The corresponding risks of macroalbuminuria were 14.4 % (95 % CI 10.8–17.9, P < 0.0001), 6.1 % (3.5–8.6, P = 0.082) and 4.7 % (3.0–6.4), respectively. The 12-year cumulative risk of ESRD was 10.3 % (95 % CI 8.4–12.4, P < 0.0001) for current smokers and 10.0 % (7.9–12.3, P < 0.0001) for ex-smokers, compared with 5.6 % (4.6–6.7) for nonsmokers. In the current smokers, one pack-year increased the risk of macroalbuminuria with a HR of 1.025 (1.010–1.041) and the risk of ESRD with a HR of 1.014 (1.001–1.026) compared with nonsmokers, in the fully adjusted model. In the ex-smokers, the risk of macroalbuminuria and ESRD was no different from the risk in nonsmokers after multivariable adjustment.
Conclusions
Current smoking is a risk factor for the progression of diabetic nephropathy and the risk increases with the increasing dose of smoking. Ex-smokers seem to carry a similar risk of progression of diabetic nephropathy as nonsmokers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarette smoking is undoubtedly one of the most significant environmental risk factors for premature death and excess morbidity. Two recent population-based studies from the US and the UK estimated that smoking reduces overall life expectancy by at least 10 years [1, 2]. These studies also showed that more than half of the deaths among smokers are caused by smoking itself, a phenomenon mainly due to the increased risk of cardiovascular disease (CVD) and cancer. However, part of the excess morbidity maybe explained by the deleterious effect on the kidneys. Large population-based observational studies have reported up to 30 % increase in the risk of chronic kidney disease (CKD) in smokers compared with nonsmokers [3, 4].
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD), the most severe form of chronic kidney disease, requiring dialysis or kidney transplantation [5]. Poor glycaemic control, high blood pressure, duration of diabetes, male sex, microalbuminuria and an unfavourable lipid profile are known risk factors for the development of diabetic nephropathy in patients with type 1 diabetes [6–8]. Smoking is also considered to have harmful effects that contribute to the development of diabetic nephropathy [9]. However, the data regarding the effects of smoking on the initiation and development of diabetic nephropathy have been all but consistent. Older studies, in relatively small number of patients with type 1 diabetes, showed that smoking is associated with an increased risk of nephropathy [10–12], but in more recent larger prospective studies, the results have been inconsistent. A study from the USA showed that current smokers have a higher risk of microalbuminuria during a 4-year follow-up [13]. A Danish prospective study showed that smoking is associated with the progression of albuminuria [14]. However, two other Danish prospective studies from the same group at the Steno Diabetes Center showed contradictory results: while in one smoking was not associated with the decline in kidney function or the progression of albuminuria, in the other smoking was not shown to be a risk factor for the development of microalbuminuria [15, 16]. It is also of note that although smoking was associated with micro- and macroalbuminuria in the original EURODIAB study [17], smoking was not associated with the progression of diabetic nephropathy in the EURODIAB prospective study [18]. A follow-up study from Switzerland showed that smoking was associated with poorer glycaemic control and progression to macroalbuminuria, but not with the development of microalbuminuria [19]. Finally, a German study showed that although smoking is associated with the decline in kidney function it does not increase the risk of proteinuria [20].
Given that the true effect of smoking on the development of diabetic nephropathy is still unresolved and a matter of debate, we decided to further explore the effect of smoking on the progression of diabetic nephropathy and on the development of its most severe form, end-stage renal disease, in one of the largest cohorts of patients with type 1 diabetes with and without nephropathy, namely the prospective Finnish Diabetic Nephropathy Study (FinnDiane).
Methods
The study population consisted of participants from the FinnDiane study, an ongoing, nationwide, prospective, multicentre study, initiated in order to find genetic and environmental risk factors for diabetic micro- and macrovascular complications, with special emphasis on diabetic nephropathy. Participating study centres included all five university hospitals, all 16 central hospitals, the majority of the regional hospitals and several major healthcare centres in Finland. Patients with type 1 diabetes were recruited at their regular outpatient visits. All patients gave their informed and written consent. The study protocol is in accordance with the principles of the Declaration of Helsinki as revised in 2000 and was approved by the local ethics committees at each study centre.
At the baseline visit, the patients underwent a thorough physical examination, blood samples were drawn, and a wide variety of metabolic markers were analysed [21]. Patients filled in questionnaires regarding their lifestyle, including their present or former smoking habits. Patients smoking at least one cigarette per day for at least 1 year were considered smokers, and patients who had stopped smoking were considered ex-smokers. If the patients had started smoking the same year they attended their baseline visit, they were excluded from the study. Nonsmokers included only patients who had never smoked. The cumulative amount of smoking was calculated in pack-years. The definition for one pack-year equals smoking 20 cigarettes per day during 1 year.
The renal status was based on two out of three consecutive overnight or 24-h urine collections. The definition of normoalbuminuria was a urinary albumin excretion rate (UAER) less than 20 µg/min (overnight) or 30 mg/24 h, for microalbuminuria a UAER ≥20 <200 µg/min (overnight) or ≥30 <300 mg/24 h and for macroalbuminuria a UAER ≥ 200 µg/min or ≥300 mg/24 h. ESRD was defined as patients on dialysis or patients having undergone kidney transplantation.
The estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [22]. The estimated glucose disposal rate (eGDR) was calculated with an equation modified for the use of HbA1c instead of HbA1, (eGDR = 24.4–12.97 × WHR − 3.39 × AHT − 0.60 × HbA1c), where WHR stands for waist-to-hip ratio and AHT for antihypertensive treatment or blood pressure ≥140/90 mmHg (yes = 1, no = 0) [23]. Patients were divided into six different social classes according to their education and employment status. For the analyses, the highest two social classes were compared with the other groups.
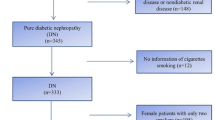
During follow-up, renal status was assessed by review of all recorded UAER values from the available medical files. The follow-up data for ESRD were also based on the information from The Care Register for Health Care and The Drug Prescription Register until year 2013. The change in renal status was verified based on at least two out of three consecutive urinary collections. The follow-up of the patients started at the baseline visit and ended when a diagnosis of the complication in question (micro-, macroalbuminuria or ESRD) was made, death from any cause had occurred or re-examination of the patient or the latest screening of their medical files had been performed. Follow-up data for ESRD were available for 3613 patients with data on smoking status (current, ex-, or nonsmoker) and 3411 patients with more precise smoking data on pack-years. Data for the development of earlier stages of nephropathy were available for 3305 patients with smoking status and 3121 patients with pack-year data. The risk of development of macroalbuminuria was calculated for patients with baseline normal UAER or microalbuminuria, and the risk of ESRD was calculated for the whole population.
Patients were divided into different groups according to their smoking status. The baseline characteristics are presented as means (±standard deviation, SD) for normally distributed values, otherwise as medians (interquartile range). Categorical variables are reported as percentages. Differences between the groups were analysed by ANOVA for normally distributed continuous variables, otherwise by the Kruskal–Wallis test. Categorical variables were analysed using the χ 2 test.
The 12-year cumulative incidence of microalbuminuria and macroalbuminuria in the groups with different smoking status was estimated using the Kaplan–Meier method, and the log-rank test was used to test the differences between the groups. The 12-year cause-specific cumulative incidence of ESRD was calculated by using the Fine and Gray’s test with death as competing risk. The cumulative effect of smoking was analysed by Cox regression models with pack-year as continuous variable providing hazard ratios (HRs) with 95 % CI for the development of different stages of diabetic nephropathy. In the multivariable models, the results were adjusted for sex, duration of diabetes, HbA1c, systolic blood pressure, HDL-cholesterol, triglycerides, BMI and social class. The effect of smoking cessation on the development of diabetic nephropathy in ex-smokers was analysed by Cox regression models using the years between smoking cessation and baseline visit as continuous variable. The assumption of the proportional hazards was tested by plotting Schoenfeld residuals against time and testing a nonzero slope by including time–covariate interaction. Finally, restricted cubic spline graphs were used to graphically evaluate the relationship between continuous measure of pack-years and the combined any progression of diabetic nephropathy or incident ESRD. P values < 0.05 were considered statistically significant. IBM SPSS statistics version 22 (IBM Corporation, Armonk, NY, USA) and SAS 9.3 version (SAS Institute Inc, Cary, NC) were used for all statistical analyses.
Results
The overall follow-up time was 15,960 person-years for the development of microalbuminuria (median follow-up time 6.5, IQR 4.3–10.1), 19,263 person-years for macroalbuminuria (median 6.5 IQR 4.3–10.0) and 42,090 person-years for ESRD (median 12.2, IQR 10.0–14.0). Table 1 presents the clinical characteristics by smoking status at baseline. The percentages of current smokers, ex-smokers and nonsmokers were 25.8, 20.3 and 53.9 %, respectively. Ex-smokers were older and had the longest duration of diabetes. Current smokers had the highest HbA1c and insulin dose and had together with the ex-smokers lower eGDR than the nonsmokers, indicating that smokers have poorer glycaemic control and are more insulin resistant. The nonsmokers had the most favourable lipid profiles with the highest HDL-cholesterol and the lowest LDL-cholesterol and triglyceride concentrations. The percentage of patients with micro- or macroalbuminuria was higher among the current and the ex-smokers compared with the patients who had never smoked. The number of pack-years was higher in current smokers compared with the patients who had quitted smoking.
The 12-year cumulative risk of microalbuminuria was 18.9 % (95 % CI 14.6–23.0, P < 0.001) for current smokers and 15.1 % (10.3–19.6, P = 0.087) for ex-smokers, compared with 10.0 % (7.8–12.1) for nonsmokers. The percentages for macroalbuminuria were 14.4 % (95 % CI 10.8–17.9, P < 0.0001), 6.1 % (3.5–8.6, P = 0.082) and 4.7 % (3.0–6.4), respectively. The 12-year cumulative risk of ESRD was 10.3 % (95 % CI 8.4–12.4, P < 0.0001) for current smokers and 10.0 % (7.9–12.3, P < 0.0001) for ex-smokers, compared with 5.6 % (4.6–6.7) for nonsmokers. Figure 1 depicts the cumulative risk of different stages of diabetic nephropathy.
Tables 2, 3 and 4 present the results of the Cox regression analysis for the development of different stages of diabetic nephropathy according to pack-years. Current smokers had a higher risk of developing microalbuminuria with a HR of 1.024 (1.008–1.041) compared with nonsmokers, and after adjustments for sex, duration of diabetes, HbA1c, systolic blood pressure, HDL-cholesterol, triglycerides and BMI, the HR was 1.021 (1.001–1.042). However, after the adjustment for social class the difference was no longer significant. In ex-smokers, the HR for microalbuminuria was 1.034 (1.014–1.054) in the fully adjusted model. The risk of macroalbuminuria was higher in current smokers, and the HR was 1.025 (1.010–1.041) in the fully adjusted model. In contrast, the risk of developing macroalbuminuria was no different in ex-smokers compared with nonsmokers after adjustment for sex, duration, HbA1 and systolic blood pressure.
In the Cox regression analysis for the development of ESRD, one pack-year increased the risk of ESRD in current smokers with a HR of 1.035 (95 % CI 1.025–1.045), and in the fully adjusted model, the HR was 1.014(1.001–1.026). For ex-smokers, one pack-year increased the risk of progression with a HR of 1.026 (1.015–1.038) in the unadjusted model. In the multivariable models, the risk in ex-smokers was attenuated and was no longer significantly different from nonsmokers.
When analysing the effect of smoking cessation in ex-smokers, the risk of developing macroalbuminuria was reduced with a HR of 0.931 (0.888–0.977) compared with current smokers, per each year without smoking before the baseline visit. The risk of microalbuminuria and ESRD was also reduced in ex-smokers, but the differences were no more significant after all adjustments. The Cox regression results for ex-smokers are presented in supplementary Table S1.
Figure 2a, b shows the restricted cubic splines for the HRs for any progression of diabetic nephropathy and the development of ESRD as a function of continuous measure of pack-years for current smokers. The risk was increased with the increasing amount of pack-years, and at 25-pack-years, the risk of any progression of diabetic nephropathy was 1.8 times higher and the risk of ESRD was 2.6 times higher in current smokers compared with nonsmokers.
Discussion
In this large observational study of patients with type 1 diabetes, we were able to show that cumulative smoking is associated with the development of different stages of diabetic nephropathy. In current smokers, the 12-year cumulative risks of microalbuminuria (18.9 %), macroalbuminuria (14.4 %) and ESRD (10.3 %) were higher compared with nonsmokers (10.0, 4.7 and 5.6 %, respectively). In current smokers, each pack-year increased the risk of macroalbuminuria by 2.5 % and risk of ESRD with 1.4 % compared with nonsmokers. The risk of microalbuminuria was also higher in current smokers compared with nonsmokers, although the difference was not significant after adjustments for other risk factors for diabetic nephropathy.
The 12-year cumulative risk of microalbuminuria and macroalbuminuria was not significantly higher in ex-smokers compared with nonsmokers (15.1 vs. 10.0 % and 6.1 vs. 4.6 %). However, the 12-year cumulative risk of ESRD was significantly higher in ex-smokers compared with nonsmokers (10.0 versus 5.6 %). In the Cox regression analyses, the risk of microalbuminuria was higher in ex-smokers compared with nonsmokers after all the adjustments. However, in the analyses for the risk of macroalbuminuria and ESRD the difference between ex- and nonsmokers was attenuated after adjustments for duration of diabetes and HbA1c, and the difference was no longer significant. This indicates that the risk of diabetic nephropathy in ex-smokers is no different from the risk in nonsmokers with similar duration of diabetes and glucose control. Based on these data, it is obvious that smoking cessation has a beneficial effect on the development of diabetic nephropathy as the risk of macroalbuminuria was decreased by 6.9 % per each year without smoking before the baseline visit compared with the patients who were current smokers at baseline.
Compared with previous prospective studies, our study had a much larger sample size. Many of the older studies included a rather small number of patients and had a short follow-up time [9–12]. Although the original EURODIAB study included a similar amount of patients as our study, the analyses were only cross-sectional and it is of note that in the smaller follow-up study smoking was not a risk factor for macroalbuminuria [17, 18]. Our results are, however, very similar to the data observed by the Danish prospective study with a 10-year follow-up that showed that current smokers are at higher risk of micro- and macroalbuminuria. It is, however, of note that the ex-smokers were not separated from the current or nonsmokers in that study [14]. The reason why Hovind et al. [15] did not see an association between smoking and an increase in albuminuria in patients who already had micro- or macroalbuminuria, may be the rather small number of patients studied (176 smokers and 94 nonsmokers) and shorter follow-up time (7 years) compared with our follow-up time for ESRD of 12 years. Another prospective study by Hovind et al. [16] including 277 patients with normal UAER observed that smoking was not associated with the development of microalbuminuria during 18-year follow-up. Also in our study, the risk of microalbuminuria was not significantly higher in the current smokers than in the nonsmokers after all the multivariable adjustments. However, there was a trend towards a higher risk of microalbuminuria in the current smokers.
A number of mechanisms have been suggested to explain the increased risk of diabetic nephropathy in smokers. Diabetic nephropathy is a disease of the small blood vessels, and smoking has harmful effects on the vasculature through direct effects on the endothelium, prothrombotic factors, platelet activation, oxidative stress and inflammation [24]. In patients with diabetes, smoking is also affecting the glucose metabolism and is associated with worse glycaemic control and insulin resistance [19, 25–30]. In our study, the current smokers had higher baseline HbA1c and lower eGDR compared with nonsmokers, and their daily insulin dose was also higher. Therefore, impaired glycaemic control and insulin resistance are likely to be key factors contributing to the deleterious effects of smoking. Smokers had also a less favourable lipid profile with higher triglyceride concentrations, which are by themselves associated with the progression of diabetic nephropathy [31]. The increased risk of progression of diabetic nephropathy in the current and the ex-smokers was attenuated after adjustments for HbA1c and the lipid variables, indicating that a part of the harmful effects of smoking is in fact mediated through glucose control and lipids.
The main strength of this study is the large number of patients with comprehensive baseline and follow-up data on the progression of diabetic nephropathy. However, our study has some limitations. Smoking status was based on self-reported questionnaires, and no laboratory measurements such as serum cotinine levels were measured. This may have influenced the results, although most probably by diluting the difference between the smokers and the nonsmokers, as some smokers could have reported being nonsmokers or ex-smokers. We did not have comprehensive data on smoking status during follow-up, and thus, some patients may have ceased or started smoking during the study period. Although the overall follow-up time for the progression of nephropathy was long, the individual follow-up time for some patients was rather short, and this may affect the risk of progression in these patients. However, in the analyses for ESRD the follow-up time was long enough in the whole study population.
Although the overall smoking prevalence has decreased during the last decades in Finland, 22 % of men and 15 % of women are still smoking. The proportion of smoking men has decreased steadily from the 1980s, but the proportion of smoking women has after a steady increase started to decrease only during the last few years [32]. It is of note that almost half of the patients with type 1 diabetes in the FinnDiane Study are current or ex-smokers, and given the obvious health hazards related to smoking and the obvious increased risk of progression of diabetic nephropathy, it is of outmost importance to counsel patients to quit smoking.
To our knowledge, this is the first study to address the association between cumulative smoking and the development of diabetic nephropathy using continuous pack-year data. Based on our results, we can conclude that smokers carry a higher risk of progression of diabetic nephropathy, and this risk increases with the increasing cumulative dose of smoking. However, in ex-smokers, the risk of progression of nephropathy decreases after smoking cessation and gradually equalizes with the risk seen in nonsmokers. The increased risk of macroalbuminuria and ESRD in current smokers was seen also after the most important other risk factors for diabetic nephropathy were taken into account, and therefore, the results indicate that smoking is a true and independent risk factor for the development of diabetic nephropathy.
Abbreviations
- UAER:
-
Urinary albumin excretion rate
- eGFR:
-
Estimated glomerular filtration rate
- eGDR:
-
Estimated glucose disposal rate
- ESRD:
-
End-stage renal disease
- FinnDiane:
-
Finnish Diabetic Nephropathy Study
- IQR:
-
Interquartile range
References
Jha P, Ramasundarahettige C, Landsman V et al (2013) 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 368:341–350
Pirie K, Peto R, Reeves GK, Green J, Beral V, Million Women Study C (2013) The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381:133–141
Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J (2003) Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14:2934–2941
Hallan SI, Orth SR (2011) Smoking is a risk factor in the progression to kidney failure. Kidney Int 80:516–523
U.S. Renal Data System (2013) USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
Jacobsen P, Rossing K, Tarnow L et al (1999) Progression of diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int Suppl 71:S101–S105
Jenkins AJ, Lyons TJ, Zheng D et al (2003) Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int 64:817–828
Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2003) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290:2159–2167
Chase HP, Garg SK, Marshall G et al (1991) Cigarette smoking increases the risk of albuminuria among subjects with type I diabetes. JAMA 265:614–617
Muhlhauser I, Sawicki P, Berger M (1986) Cigarette-smoking as a risk factor for macroproteinuria and proliferative retinopathy in type 1 (insulin-dependent) diabetes. Diabetologia 29:500–502
Sawicki PT, Didjurgeit U, Muhlhauser I, Bender R, Heinemann L, Berger M (1994) Smoking is associated with progression of diabetic nephropathy. Diabetes Care 17:126–131
Couper JJ, Staples AJ, Cocciolone R, Nairn J, Badcock N, Henning P (1994) Relationship of smoking and albuminuria in children with insulin-dependent diabetes. Diabetes Med 11:666–669
Scott LJ, Warram JH, Hanna LS, Laffel LM, Ryan L, Krolewski AS (2001) A nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetes. Diabetes 50:2842–2849
Rossing P, Hougaard P, Parving HH (2002) Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 25:859–864
Hovind P, Rossing P, Tarnow L, Parving HH (2003) Smoking and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 26:911–916
Hovind P, Tarnow L, Rossing P et al (2004) Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 328:1105
Chaturvedi N, Stephenson JM, Fuller JH (1995) The relationship between smoking and microvascular complications in the EURODIAB IDDM Complications Study. Diabetes Care 18:785–792
Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N (2004) Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 47:1020–1028
Gerber PA, Locher R, Schmid B, Spinas GA, Lehmann R (2013) Smoking is associated with impaired long-term glucose metabolism in patients with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis 23:102–108
Orth SR, Schroeder T, Ritz E, Ferrari P (2005) Effects of smoking on renal function in patients with type 1 and type 2 diabetes mellitus. Nephrol Dial Transplant 20:2414–2419
Thorn LM, Forsblom C, Fagerudd J et al (2005) Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 28:2019–2024
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ (2000) Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 49:626–632
Barua RS, Ambrose JA (2013) Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol 33:1460–1467
Nilsson PM, Gudbjornsdottir S, Eliasson B, Cederholm J, Steering Committee of the Swedish National Diabetes Register (2004) Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes–data from the National Diabetes Register in Sweden. Diabetes Metab 30:261–268
Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM (1992) Insulin resistance and cigarette smoking. Lancet 339:1128–1130
Axelsson T, Jansson PA, Smith U, Eliasson B (2001) Nicotine infusion acutely impairs insulin sensitivity in type 2 diabetic patients but not in healthy subjects. J Intern Med 249:539–544
Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH (2009) Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 58:2220–2227
Bergman BC, Perreault L, Hunerdosse D et al (2012) Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61:3156–3166
Seet RC, Loke WM, Khoo CM et al (2012) Acute effects of cigarette smoking on insulin resistance and arterial stiffness in young adults. Atherosclerosis 224:195–200
Tolonen N, Forsblom C, Thorn L et al (2009) Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia 52:2522–2530
Helakorpi S, Holstila A, Virtanen S, Uutela A (2011) Health Behaviour and Health among the Finnish Adult Population, Spring 2011. National Institute for Health and Welfare (THL). http://urn.fi/URN:ISBN:978-952-245-566-6. Accessed November 2012
Acknowledgments
The authors would like to acknowledge all physicians and nurses at each FinnDiane Center participating in patient recruitment and characterization (see online supplement).
Funding
This research was supported by grants from the Folkhälsan Research Foundation, Academy of Finland (134379), Wilhelm and Else Stockmann Foundation, Diabetes Research Foundation, Liv och Hälsa Foundation, Finska Läkaresällskapet, NovoNordisk Foundation, Signe and Ane Gyllenberg Foundation and Päivikki and Sakari Sohlberg Foundation. The funding sources were not involved in the design or conduct of the study.
Author contribution
MF designed the study, carried out statistical analyses and wrote the manuscript. VH contributed to the design of the study, statistical analyses and aspects of the manuscript. CF contributed to the design of the study and critically revised the manuscript. LT, JW, NT and RL contributed to the collection of the data and critically revised the manuscript. P-HG contributed to the study design and edited and critically revised the manuscript. P-HG is the guarantor of this work, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P.-H. G. has received lecture honorariums from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Genzyme, Medscape, MSD, Novartis, Novo Nordisk and Sanofi. P.-H. G. is an advisory board member of Abbott, AbbVie, Boehringer Ingelheim, Cebix, Eli Lilly, Janssen, Medscape and Novartis. P.-H. G. has received investigator-initiated study grants from Eli Lilly and Roche. No other potential conflicts of interest relevant to this article were reported.
Ethical standard
The study was conducted in accordance with the Declaration of Helsinki and accepted by the Helsinki University Hospital Research Ethics Committee.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Massimo Federici.
On behalf of the FinnDiane Study Group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feodoroff, M., Harjutsalo, V., Forsblom, C. et al. Smoking and progression of diabetic nephropathy in patients with type 1 diabetes. Acta Diabetol 53, 525–533 (2016). https://doi.org/10.1007/s00592-015-0822-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0822-0