Abstract
Aims
Dietary fats have been shown to promote the translocation of bacterial endotoxins from the gut into circulation, which may induce systemic inflammation and modulate the inflammatory response of circulating immune cells. The aim of this study was to determine the effect of the postprandial milieu on inflammation and the inflammatory response of antigen presenting cells in the context of type 1 diabetes (T1D).
Materials and methods
Eleven patients with T1D and eleven nondiabetic controls were recruited as part of the FinnDiane study and given two fatty meals during 1 day. Cytokine responses in monocytes and myeloid dendritic cells (mDCs) as well as serum lipopolysaccharide activity levels, triglyceride concentrations and cytokine concentrations were measured from fasting and postprandial blood samples.
Results
Postprandially, patients with T1D and controls showed significant increases in eight inflammatory cytokines (IL-6, TNF-α, IL-1β, IFN-α, IL-10, IFN-γ, IL-12 and MIP-1β) without concomitant increase in serum LPS activity. Serum cytokine production was similar in both groups. No postprandial change was seen in the IL-6, TNF-α or IL-1β production of mDCs or monocytes. At fasting, diabetic mDCs exhibited higher LPS-induced IL-6 and IL-1β production than controls.
Conclusions
Acute high-fat meals increase circulating cytokines but have no effect on serum lipopolysaccharide activity levels or cytokine production in circulating mDCs or monocytes. Our results suggest that postprandial increase in serum cytokine levels is neither mediated by circulating endotoxins nor the activation of circulating innate cells. The production of high-fat meal-induced inflammatory markers is most likely regulated at the tissue level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary fat is known to increase circulating levels of bacterial lipopolysaccharide (LPS) and proinflammatory cytokines in healthy subjects [1–3]. Earlier studies have found increased intestinal permeability in patients with type 1 diabetes (T1D), suggesting that patients may display a more pronounced increase in circulating endotoxins after the ingestion of a high-fat meal [4]. LPS is a unique, evolutionarily conserved component of the outer membrane of Gram-negative bacteria, commonly used to model innate immune responses to bacterial infection. In murine and cell culture models, fatty acids have been shown to modulate LPS-induced proinflammatory cytokine secretion, notably IL-6 and tumor necrosis factor (TNF)-α [5, 6]. While it has been hypothesized that a postprandial surge of bacterial LPS in peripheral blood elicits the commonly reported increases in inflammation, elevated concentrations of circulating fatty acids may also play a role in postprandial inflammation [5, 7–10]. Indeed, a number of earlier studies have reported TLR4/NF-κB-mediated inflammation in response to fatty acids; however, the precise sources and causes of postprandial inflammation remain unclear [5, 10, 11].
The transmembrane signaling protein toll-like receptor 4 (TLR4) is found on monocyte lineage immune cells and facilitates LPS-induced inflammation and cellular activation, primarily through the NF-κB signaling pathway [12]. One earlier study reported abnormally high TLR4 expression on the surface of monocytes isolated from patients with T1D [13]. Similarly, in vitro studies have shown isolated monocytes and myeloid dendritic cells (mDCs) from T1D patients to be more sensitive to LPS than those isolated from healthy controls [14]. Furthermore, we have recently shown LPS activity levels to be strongly associated with the components of the metabolic syndrome, concentrations of circulating inflammatory markers and the development of diabetic nephropathy in patients with T1D [15–17]. Increased TLR4 responsiveness together with postprandially elevated circulating LPS and triglycerides levels may thus play a key role in the promotion and progression of inflammation, thereby increasing the risk of developing diabetic complications [18].
The aim of the present study was to investigate the acute effects of two sequential high-fat meals on circulating cytokines and the LPS responsiveness of innate immune cells in the context of T1D. We hypothesized that high-fat diet-induced endotoxemia would modulate TLR4 responsiveness and increase cytokine release from peripheral innate immune cells, contributing to postprandial systemic inflammation, particularly in patients with T1D.
Materials and methods
Study subjects
Eleven patients with T1D and 11 nondiabetic controls participated in an oral fat load test organized by the Finnish Diabetic Nephropathy Study (FinnDiane; www.finndiane.fi). The study subjects fulfilled the following selection criteria: (1) age between 20 and 65 years, (2) nonsmokers, (3) no use of antibiotics during the past month, (4) no gut related illnesses, (5) no diarrhea during the past month and (6) no traveling outside of the Nordic countries during the past month. Type 1 diabetes was defined as diagnosis of diabetes before the age of 40 and permanent insulin treatment initiated within 1 year of diagnosis. None of the patients were taking lipid lowering fibrates or omega-3 supplements. The study protocol was approved by the local ethics committee and was in accordance with the Declaration of Helsinki. All participants gave given written informed consent to their participation in the study.
Oral fat load
During the examination day, the participants were given two energy-rich, high-fat meals: breakfast at 8:00 (965 kcal, 65 g fat) and lunch at 12:00 (870 kcal, 42 g fat). Energy (E%) content of oral fat loads was as follows: breakfast (proteins 19 E%, carbohydrates 24 E%, fats 57 E%) and lunch (proteins 11 E%, carbohydrates 39 E%, fats 50 E%).
Laboratory analyses
Blood samples were taken after an overnight fast at 8:00 and again at 14:00. Plasma insulin concentrations were analyzed with an immunofluorometric assay (AutoDelfia, Wallac, Finland). C-reactive protein (hsCRP) was measured from serum using a high-sensitivity immunoprecipitation assay (Thermo Scientific, Vantaa, Finland). Serum lipid and lipoprotein concentrations were determined with a Konelab analyzer using automated enzymatic methods (Thermo Scientific) in the hospital laboratory (Division of Cardiology, Biomedicum, Helsinki). Serum LPS activity was measured with the Limulus amoebocyte lysate (LAL) chromogenic assay (Hycult Biotechnology, Uden, the Netherlands; intra-assay coefficients of variation, 4.5 %). Other laboratory tests including blood cell counts were performed in the Helsinki University Central Hospital Laboratory (HUSLAB; www.hus.fi).
Flow-cytometric analysis of circulating cytokines
Serum concentrations of 13 circulating cytokines and chemokines (IL-6, TNF-α, IL-1β, IFN-α, IL-10, IFN-γ, IL-12p70, MIP-1β, IL-1α, IP-10, MIP-1α, IL-8 and MCP-1) were assayed using the FlowCytomix Multiple Analyte System (eBioscience, Vienna, Austria) according to the manufacturer’s instructions. Data were analyzed using FlowcytomixPro (eBioscience, Vienna, Austria) and Prism (GraphPad Software, Inc., La Jolla, Ca, USA). Standard curves were generated, and concentrations calculated using Prism.
Intracellular cytokine staining and LPS stimulation
Immediately after collection, 200 µl of sodium heparin blood was incubated with or without 100 ng/ml E. coli LPS (Strain 0111:B4; DIFCO 3122-25-8 Detroit, MI) for 4 h at 37 °C and 5 % CO2. After the first hour, 1.2 µl of 1:10 diluted GolgiStop (BD Biosciences, San Jose, CA, USA) was added. After the incubation, blood was mixed with surface marker antibodies (CD14 PE-Cy5.5, CD19 PE-Cy5.5 and CD1c APC) and incubated for 20 min in the dark at room temperature (RT). The red blood cells were then lysed by incubating whole blood in 2 ml of FACS Lysing Solution (BD Biosciences) for 10 min, RT. The white blood cells were pelleted out of solution by centrifugation (500 g for 5 min, RT) and washed twice with a washing solution of 5 % heat-inactivated fetal calf serum (0.22 µm sterile filtered) and 0.02 % NaN3 in PBS. The washed cells were fixed with 4 % paraformaldehyde in PBS and permeabilized using BD Perm/Wash according to the manufacturer’s instructions and stained for intracellular cytokines IL-1β FITC, TNF-α-PE, and IL-6 PE or isotype controls. Staining controls were prepared in the same way using 100 µl of blood. A full list of antibodies can be found in the supplementary materials.
Data analysis and statistical methods
Cytokine expression was reported as percent expressing or responding (for gating strategy, refer to Supplementary Fig. 1). Percent expressing refers to the percentage of cells expressing a particular cytokine. Percent responding is calculated by taking the percent of cells expressing a particular cytokine after incubation with LPS and subtracting the percent of cells expressing the same cytokine at the same time point in the sample incubated without LPS. Subtraction was performed to control for baseline expression and accurately determine the cytokine expression specifically induced by exogenous LPS incubation. The maximum number of events (300,000–500,000) was collected for each sample. The raw flow cytometry data were analyzed using FlowJo (Treestar, Inc., San Carlos, CA, USA).
Statistical analyses were performed using SPSS (v.19; IBM Armonk, NY, USA). The Shapiro–Wilk test was performed to determine normality. The Mann–Whitney U and Wilcoxon signed-rank tests were used to determine group effect. Pearson’s and Spearman’s correlation coefficients were used to determine the relationship between measured variables. For all statistics, p values of <0.05 were considered statistically significant.
Results
Clinical characteristics of the study subjects
The clinical characteristics of 11 nondiabetic controls and 11 patients with T1D are shown in Table 1. Controls had higher BMI and higher fasting plasma triglycerides than patients with T1D. As expected, patients with T1D had higher HbA1c and fasting serum HDL cholesterol concentrations than controls. At the postprandial state, differences between plasma triglycerides and HDL concentrations remained significant between the groups (Table 1). The controls showed a more pronounced increase in postprandial triglycerides compared to patients with T1D (120 ± 59 vs. 72 ± 42 %; p = 0.023). Although only control subjects showed moderate increases in the endotoxin activity levels during the day, the serum LPS activity levels did not significantly differ between the groups at either the fasting or postprandial state (Table 1). No significant group differences were seen in fasting blood cell counts (Table 2).
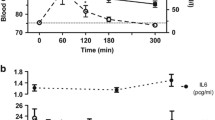
Patients with T1D showed a significant postprandial increase in IL-6, TNF-α, IL-1β, IFN-α, IFN-γ and macrophage inflammatory protein (MIP)-1α (Fig. 1, Supplementary Table 1). Controls showed a significant increase in IL-6, TNF-α, IL-1β, IFN-α, IL-10 and IL-12. No significant differences were found between the two groups at either time point. When the groups were combined, we observed significant postprandial increases in the concentrations of 8 out of the 13 innate immune-derived cytokines: IL-6, TNF-α, IL-1β, IFN-α, IL-10, IFN-γ, IL-12, MIP-1β and IL-1α (Fig. 1, Supplementary Table 1). No correlations were found between serum LPS activity levels and circulating cytokine concentrations. At the postprandial state, plasma triglycerides were positively associated with circulating IL-12 (r = 0.574, p = 0.013) and IL-10 (r = 0.491, p = 0.028) concentrations. Postprandial HDL cholesterol concentrations were inversely correlated with circulating IL-10 (r = −0.449, p = 0.030) and IP-10 (r = −0.519, p = 0.016).
Circulating cytokine concentrations at the fasting and postprandial states. Triangles and circles indicate median values for nondiabetic controls and patients with T1D, respectively. Values are pg/ml of cytokine. Bars represent the inter quartile range. Asterisks indicate a significant difference between 8:00 (fasting) and 14:00 h (postprandial) (p < 0.05). Grouped p values represent the significance of the difference between 8:00 and 14:00 when the patient and control samples are grouped
Flow-cytometric analysis of IL-6, TNF-α and IL-1β cytokine production in unstimulated blood monocyte and dendritic cells.
At the fasting state, baseline monocyte TNF-α, IL-6 and IL-1β production was similar in patients with T1D and nondiabetic controls (Fig. 2). Postprandially, CD14 + monocytes from patients with T1D showed lower levels of spontaneous IL-6 expression compared to controls (8 vs. 10 % expressing, p = 0.008). Compared to controls, fasting CD1c + mDCs from patients with T1D exhibited higher levels of TNF-α (11 vs. 5 % expressing, p = 0.01) and IL-1β (9 vs. 6 % expressing, p = 0.02). At the postprandial state, we observed no differences between patient and control mDC cytokine production (Fig. 2).
Cytokine secretion at fasting and postprandial state. Triangles and circles indicate median values for nondiabetic controls and patients with T1D, respectively. Empty symbols represent samples incubated ex vivo. Filled symbols represent samples incubated ex vivo in the presence of LPS. Values are percent of cells expressing the cytokine. Bars represent the interquartile range. Asterisks indicate significant differences between the groups (p < 0.05)
Flow-cytometric analysis of IL-6, TNF-α and IL-1β cytokine production in LPS-stimulated blood monocyte and dendritic cells.
To investigate the effects of bacterial endotoxin-induced inflammatory responses at the fasting and postprandial states, we measured cytokine production (IL-6, TNF-α and IL-1β) in monocytes and mDCs after stimulation with E. coli LPS. Notably, there were no significant differences between the T1D patients and healthy controls in the LPS-induced monocyte cytokine production either at the fasting or at the postprandial state. At the fasting state, the LPS-stimulated mDCs from patients with T1D exhibited higher IL-6 (p = 0.034) and IL-1β (p = 0.040) production than in the control subjects (Fig. 2). Although there was no significant difference between the groups, the LPS-stimulated TNF-α production tended to be higher in the patients at fasting. In contrast, in the un-subtracted LPS-stimulated expression data, mDCs from fasting patients with T1D showed a significantly higher TNF-α response than mDCs from fasting controls (p = 0.031, data not shown). At the postprandial state, no group differences in cytokine production were observed in LPS-stimulated mDCs (Fig. 1, Supplementary Table 2). In the LPS-stimulated mDCs, fasting IL-6 and postprandial TNF-α production correlated negatively with HbA1c (r = −0.620, p = 0.042; and −0.644, p = 0.044, respectively) in patients with T1D.
Discussion
The results of the present study show that a high-fat diet increases the concentrations of circulating inflammatory markers similarly in patients with T1D and nondiabetic controls. However, a high-fat diet had no effect on IL-6, TNF-α or IL-1β production in circulating monocytes or mDCs. Thus, contrary to expected, our data suggest that the inflammatory effects of an acute bout of postprandial lipemia are conserved in patients with T1D and nondiabetic controls. Furthermore, our results confirm that the postprandial milieu induces the release of circulating cytokines and show that this systemic inflammation is not caused by the activation of circulating mDCs or monocytes and may thus be independent of postprandial endotoxin release.
Recent studies on postprandial increases in serum LPS activity have suggested that dietary fat-induced metabolic endotoxemia could be responsible for the commonly observed postprandial inflammation [19]. In the present study, an increase in the serum concentrations of 8 out of 13 inflammatory cytokines and chemokines was observed in response to two sequential high-fat meals. This increase in serum cytokine concentrations was not explained by a postprandial increase in circulating LPS or ex vivo cytokine production from circulating innate immune cells. Similarly, no correlation was found between circulating LPS activity and postprandial cytokine concentrations. Hence, our data suggest that the observed postprandial inflammation is not primarily induced by circulating LPS but is likely mediated by other postprandially elevated serum factors. Although the triglyceride concentrations were only correlated with two of the circulating markers of inflammation (IL-10 and IL-12), it is possible that lipemia itself may be the culprit. This view is supported by the fact that circulating fatty acids can bind to TLR4, thereby inducing inflammation via the NF-κB pathway [5, 10].
Earlier reports showing postprandial increases in TLR4 ligands (i.e., LPS and triglycerides) in addition to reports on TLR4 hyperresponsiveness in diabetic immune cells [5, 8, 9, 14, 20, 21] led us to hypothesize that patients with T1D may exhibit a dysregulated proinflammatory response to high-fat meals. We were indeed able to confirm the disease-related TLR4 hyperresponsiveness in mDCs, but not in monocytes. Furthermore, postprandial monocytes and mDCs showed no postprandial increase in cytokine production after an ex vivo incubation. Therefore, an early report of postprandial increases in intracellular cytokine production in monocytes could not be replicated [22]. This discrepancy may be due to methodological differences. In the earlier study, whole blood was diluted in a high glucose media, which is known to increase in vitro cytokine secretion [23]. Our results clearly demonstrate that acute fat loads do not influence cytokine production in minimally manipulated circulating monocytes or mDCs derived from nondiabetic subjects or patients with T1D. Nevertheless, postprandial cytokine production may become more relevant in individuals who have long-term exposure to dietary fats or suffer from underlying chronic diseases such as obesity, type 2 diabetes, metabolic syndrome or kidney disease.
Our data demonstrate that the inflammatory response to high-fat meals is conserved in patients with T1D and nondiabetic individuals. While there was a significant postprandial surge in the circulating IL-6, TNF-α, IL-1β, MIP-1α, IL-10, IFN-α, IFN-γ and IL-12 concentrations, the expected diabetes-related hypersensitivity to the fat load was not seen in the serum or in the circulating innate immune cells. However, it is of note that postprandial inflammation may still contribute to an increased risk of diabetic complications in a diabetes-associated metabolic milieu. Indeed, there is evidence from murine models that diabetic tissues are hyperresponsive to postprandially upregulated cytokines such as TNF-α [24] and that prolonged, elevated triglyceride concentrations are able to potentiate the release of such cytokines [5, 6]. However, future research in humans is needed to determine the long-term effects of postprandial inflammation on the risk of diabetic complications.
Interestingly, only the nondiabetic controls displayed a modest, nonsignificant postprandial increase in the endotoxin activity levels. Indeed, preliminary results from our more extensive dietary intervention study suggest that patients with T1D exhibit significantly lower serum LPS activity response to the fat load when compared to nondiabetic controls [25]. Such a lower LPS response to the fat challenge could be explained by either defective transport mechanisms or increased concentrations of detoxifying factors in patients. In addition to circulating HDL, one other factor that may be differentially expressed in patients with T1D is intestinal alkaline phosphatase (IAP), which suppresses inflammation in the gut by detoxifying intestinal LPS [26]. Of note, serum concentrations of IAP have been shown to be increased in pathogenic conditions such as diabetes, liver disease and chronic renal failure [27].
We found that fasting mDCs from patients were both hyperresponsive to LPS and exhibited increased spontaneous TNF-α and IL-1β cytokine production when compared with healthy controls. Notably, we also found that mDCs from patients with higher HbA1c showed a trend toward a lower IL-6 and TNF-α response to LPS. This alteration suggests that the hyperresponsiveness of mDCs may not be secondary to metabolic disturbances. Instead, the data suggest that the mDC hyperreactivity could be a disease-related functional defect that may be stable over time and possibly under genetic control.
These changes in the function of mDCs may contribute to an altered T cell function in disease pathogenesis. In fact, Th-17 cells have recently been proposed to be a potential mediator of the autoimmune destruction of pancreatic β-cells. Increased numbers of Th-17 cells have been shown in the peripheral lymph nodes of adult patients with T1D as well as in the peripheral blood of children recently diagnosed with T1D [28, 29]. Although the causes of this increase in Th-17 cell numbers have yet to be elucidated, it is known that the cytokines IL-6, IL-1β and TNF-α are all involved in the proliferation and differentiation of Th-17 cells [30, 31].
In contrast to earlier studies that used isolated cells in culture media [13, 14, 20], in whole blood we were unable to the confirm reports of increased monocyte cytokine secretion in response to LPS. However, although experiments with whole blood more closely approximate the reactivity of circulating cells in vivo, it cannot be ruled out that the presence of autologous serum factors at physiological concentrations may have masked some underlying differences in the monocyte LPS response in patients with T1D [32]. Moreover, it is possible that this discrepancy could be due to elevated concentrations of LPS-binding proteins or circulating compensatory factors such as serum lipids in the peripheral blood of patients with T1D. Indeed, compared to healthy controls, the patients showed higher fasting serum HDL cholesterol, a factor known to buffer LPS-induced inflammation [33].
Previous studies investigating postprandial inflammation have primarily focused on circulating inflammatory markers rather than cell-specific inflammation [34]. This is indeed the first study to focus on the effects of a high-fat diet on circulating innate immune cells and in the context of autoimmune diabetes. Our experimental design allowed us to investigate systemic as well as intracellular inflammation in order to help elucidate the mechanisms of postprandial inflammation. Altogether, our results clearly demonstrate that high-fat meals increase circulating cytokines and chemokines and that this increase is not mediated by postprandial LPS nor circulating innate immune cells. These results suggest that the commonly observed postprandial increase in inflammatory markers is not induced by increased amounts of systemic LPS, but reflects high-fat diet-induced tissue-specific inflammation. Evidence in mice suggests that this inflammation may originate in the intestine, liver or possibly in the adipose tissue [35, 36]. Thus, this represents a paradigm shift in our understanding of early postprandial inflammation [7, 19]. Future studies in order to confirm our results and to determine the source and mechanisms of postprandial inflammation may help us unravel the connection between the postprandial milieu, inflammation and the risk of diabetic complications.
References
Cani PD, Delzenne NM (2009) Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol 9(6):737–743
Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP (2009) Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59(1):172–181
Laugerette F, Vors C, Géloën A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M (2011) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 22(1):53–59
Vaarala O, Atkinson MA, Neu J (2008) The “Perfect storm” for type 1 diabetes the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 57(10):2555–2562
Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH (2005) Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 174(9):5390–5397
Huang H, Liu T, Rose JL, Stevens RL, Hoyt DG (2007) Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J Inflamm (Lond) 4:22
Erridge C, Attina T, Spickett CM, Webb DJ (2007) A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86(5):1286–1292
Alipour A, van Oostrom AJ, Izraeljan A, Verseyden C, Collins JM, Frayn KN, Plokker TWM, Elte JWF, Cabezas MC (2008) Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 28(4):792–797
Van Oostrom A, Sijmonsma T, Rabelink T, Van Asbeck B, Cabezas MC (2003) Postprandial leukocyte increase in healthy subjects. Metab Clin Exp 52(2):199–202
Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH (2003) Reciprocal modulation of toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 278(39):37041–37051
Ye J, Keller JN (2010) Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2(6):361
Zhang G, Ghosh S (2000) Molecular mechanisms of NF-κB activation induced by bacterial lipopolysaccharide through toll-like receptors. J Endotoxin Res 6(6):453–457
Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I (2008) Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 93(2):578–583
Meyers A, Shah R, Gottlieb P, Zipris D (2010) Altered toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med 88(12):1221–1231
Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop PH, Lehto M, FinnDiane Study Group (2009) Serum lipopolysaccharide activity is associated with the progression of kidney disease in finnish patients with type 1 diabetes. Diabetes Care 32(9):1689–1693
Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J et al (2011) Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34(8):1809–1815
Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V (2011) Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34(2):392–397
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46(10):1402–1407
Laugerette F, Vors C, Peretti N, Michalski M (2011) Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation. Biochimie 93(1):39–45
Nieminen JK, Vakkila J, Salo HM, Ekström N, Härkönen T, Ilonen J, Knip M, Vaarala O (2012) Altered phenotype of peripheral blood dendritic cells in pediatric type 1 diabetes. Diabetes Care 35(11):2303–2310
Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D (2012) Dysregulated toll-like Receptor-Induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes 61(10):2525–2533
Hyson DA, Paglieroni TG, Wun T, Rutledge JC (2002) Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost 8(2):147–155
Pott GB, Chan ED, Dinarello CA, Shapiro L (2009) Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol 85(5):886–895
Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci 105(26):9047–9052
Lassenius MIS, Mäkinen V-P, Fogarty CL, Peräneva L, Jauhiainen M, Pussinen P, Taskinen M-R, Kirveskari J, Vaarala O, Nieminen JK, Hörkkö S, Kangas AJ, Soininen P, Ala-Korpela M, Gordin D, Ahola AJ, Forsblom C, Groop P-H, Lehto M (2014) Patients with type 1 diabetes show signs of vascular dysfunction in response to multiple high-fat meals. Nutr Metab 11:28
Kelly CJ, Colgan SP, Frank DN (2012) Of microbes and meals the health consequences of dietary endotoxemia. Nutr Clin Pract 27(2):215–225
Kuwana T, Rosalki S (1990) Intestinal variant alkaline phosphatase in plasma in disease. Clin Chem 36(11):1918–1921
Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O (2010) IL-17 immunity in human type 1 diabetes. J Immunol 185(3):1959–1967
Ferraro A, Socci C, Stabilini A, Valle A, Monti P, Piemonti L, Nano R, Olek S, Maffi P, Scavini M (2011) Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 60(11):2903–2913
Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA (2009) Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183(7):4432–4439
Davila E, Kolls J (2010) A “Toll” for Th17 cell expansion. J Leukoc Biol 88(1):5–7
Mallone R, Mannering S, Brooks-Worrell B, Durinovic-Belló I, Cilio C, Wong F, Schloot N (2011) Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-cell workshop committee of the immunology of diabetes society. Clin Exp Immunol 163(1):33–49
Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD (1994) Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med 180(3):1025–1035
Herieka M, Erridge C (2013) High-fat meal induced postprandial inflammation. Mol Nutr Food Res 58(1):136–146
Pettersson US, Waldén TB, Carlsson P, Jansson L, Phillipson M (2012) Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7(9):e46057
Shono S, Habu Y, Nakashima M, Sato A, Nakashima H, Miyazaki H, Kinoshita M, Tsumatori G, Shinomiya N, Seki S (2011) The immunologic outcome of enhanced function of mouse liver lymphocytes and kupffer cells by high-fat and high-cholesterol diet. Shock 36(5):484–493
Acknowledgments
The study was supported by Folkhälsan Research Foundation (PHG), Wilhelm and Else Stockmann Foundation (PHG, ML, MIL, CF), Academy of Finland (134379 to PHG, 257545 to MJ), Liv och Hälsa Foundation (MIL, PHG), Waldemar von Frenckells stiftelse (MIL), Svenska kulturfonden (MIL), Kyllikki and Uolevi Lehikoinen Foundation (MIL), Finnish Cardiovascular Foundation (SH), Helsinki University Central Hospital Research Foundation (MRT), the Sigrid Juselius Foundation (MRT, PJP, SH), Diabetes Research Foundation (ML) and The Novo Nordisk Foundation (PHG, ML). We acknowledge the nurses A. Sandelin, J. Tuomikangas and T. Soppela at the Folkhälsan Institute of Genetics for their excellent contribution to this work.
Conflict of interest
Christopher L. Fogarty, Janne K. Nieminen, Lina Peräneva, Mariann I. Lassenius, Aila J. Ahola, Marja-Riitta Taskinen, Matti Jauhiainen, Juha Kirveskari, Pirkko Pussinen, Sohvi Hörkkö, Ville-Petteri Mäkinen, Daniel Gordin, Carol Forsblom, Per-Henrik Groop, Outi Vaarala and Markku Lehto declare that they have no conflict of interest.
Statement of ethical disclosure
The study protocol was approved by the local ethical committee (221/13/03/01/2009; Ethics Committee, Department of Medicine, Helsinki University Central Hospital).
Statement of human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Managed by Massimo Porta.
On behalf of the FinnDiane Study Group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fogarty, C.L., Nieminen, J.K., Peräneva, L. et al. High-fat meals induce systemic cytokine release without evidence of endotoxemia-mediated cytokine production from circulating monocytes or myeloid dendritic cells. Acta Diabetol 52, 315–322 (2015). https://doi.org/10.1007/s00592-014-0641-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0641-8