Abstract
The aim of this study was to determine whether serum fructosamine is associated with the risk of type 2 diabetes mellitus (T2DM) in a cohort of middle-age Finnish men. Fructosamine and other cardiometabolic risk factors were measured in a sample of 2,520 subjects from the population-based Kuopio Ischemic Heart Disease study. Associations between baseline fructosamine levels and risk of T2DM involved Cox-regression analyses, progressively adjusted for potential confounders and mediators. Mean baseline age and fructosamine levels were 53 years and 267 µmol/L, respectively. During a median follow-up of 23 years (interquartile range 18–25 years), 153 (6.1 %) new cases of T2DM were recorded. We observed an approximately “J-shaped” association between fructosamine values and T2DM risk, with a nadir at about 250 µmol/L. In subjects with fructosamine ≥250 µmol/L, the hazard ratios per 1 standard deviation (28 µmol/L) higher fructosamine levels resulted 1.20 (95 % CI 1.00–1.44; p = 0.046) in a model adjusted for age, body mass index, systolic blood pressure, serum high-density lipoprotein-cholesterol and family history T2DM. This association did not materially change upon further adjustment for C-reactive protein, physical activity, serum triglyceride and insulin resistance. However, no association was found when adjusting for baseline fasting plasma glucose (FPG). In this cohort of middle-age Finnish men, we found a “J-shaped” relationship between fructosamine and risk of T2DM, with a higher risk in subjects with baseline levels of fructosamine ≥250 µmol/L. Such an increased risk, however, was not independent from baseline FPG levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because the identification of subjects at risk of type 2 diabetes mellitus (T2DM) is essential for the prevention or delay of the onset of diabetes-related macro- and microvascular complications, several prognostic and risk score models have been developed to help identify subjects at higher risk [1]. These models have suggested that risk factors such as age, sex, obesity indicators [body mass index (BMI) and waist-to-hip ratio], family history of T2DM, and glycemic [fasting plasma glucose (FPG), postload glucose and glycated hemoglobin] and nonglycemic (triglycerides, high-density lipoprotein cholesterol, uric acid) biomarkers are associated with the risk of incident T2DM; however, their clinical utility has been argued as they perform suboptimally in the prediction of individual diabetes risk [1]. Therefore, the identification of novel biomarkers to develop more accurate predictive models remains a priority.
Serum fructosamine is a glycemic marker formed via nonenzymatic processes involving the glycosylation of serum proteins to form ketoamines [2]. Compared to glycated hemoglobin (HbA1c), the “gold standard” measure of glycemic control, serum fructosamine has a shorter half-life and reflects the physiology of glucose in the extracellular space, whereas HbA1c indicates glycosylation in the intraerythrocyte compartment. Thus, fructosamine is of clinical utility to show short-term glycemic control (2–4 weeks) and when underlying conditions make HbA1c interpretation difficult (i.e., anemic states, hemoglobinopathies or renal diseases) [2].
Two previous studies have investigated the relationship between fructosamine and risk of diabetes mellitus in a US community-based prospective cohort study (Atherosclerosis Risk in Communities), showing a positive association [3, 4]. However, to our knowledge, no other prospective studies have assessed such relationship in a different population; this is relevant, given the importance of the genetic background in glycosylation pathways [5]. The objective of this study was to describe the relationship between serum fructosamine and incident T2DM in a prospective cohort of middle-age Finnish men.
Research design and methods
This study was performed following the STROBE guidelines for observational studies in epidemiology [6].
Study population
The Kuopio ischemic heart disease (KIHD) risk factor study was designed to investigate risk predictors for atherosclerotic cardiovascular outcomes in a population-based sample of men from Eastern Finland. The subjects were a randomly selected sample of 3,433 men 42–60 years of age resident in the town of Kuopio or its surrounding rural communities [7]; baseline examinations were conducted between March 1984 and December 1989.
A total of 2,682 men (82.9 % of those eligible) were recruited in two cohorts. The first cohort consisted of 1,166 men who were 54 years old, enrolled from 1984 to 1986, and the second cohort included 1,516 men who were 42, 48, 54 or 60 years old, enrolled from 1986 to 1989. The baseline examinations were followed by the 4-year examination round (1991–1993) in which 1,038 men from the second cohort (88 % of those eligible) participated. At the 11-year examination round (1998–2001), all men from the second cohort were invited, and 854 men (95 % of the eligible) participated. During the 20-year examination round, all eligible participants from the first and second cohorts were invited. A total of 1,241 men (80 % of the eligible) participated.
In this study, we excluded 162 subjects with diagnosed diabetes at baseline, defined as either having regular treatment with an oral hypoglycemic agent, insulin therapy, or having treatment only with diet while also having an FPG level of at least 7.0 mmol/L. Therefore, 2,520 participants remained for the analyses. Incident case of T2DM was defined as a self-reported physician-set diagnosis and/or FPG ≥7.0 mmol/L or 2-h oral glucose tolerance test plasma glucose ≥11.1 mmol/L at re-examination rounds 4, 11 and 20 years after the baseline and by record linkage to the national hospital discharge registry and to the Social Insurance Institution of Finland register for reimbursement of medicine expenses used T2DM for the entire study period until the end of the follow-up on December 31, 2010.
Assessment of risk factors
Blood samples were taken between 8 and 10 a.m. In addition to fasting, they were instructed to abstain from drinking alcohol for at least 3 days prior and from smoking for at least 12 h. A 30-min rest period was allowed in the supine position before blood collection using vacuum tubes (Terumo Venoject; Terumo, Tokyo, Japan). No tourniquet was used during the blood collection.
The measurement of serum fructosamine concentrations was made from frozen serum samples photometrically (Boehringer Mannheim, Mannheim, Germany) by a colorimetric method in an autoanalyzer (Kone Specific, Kone inc, Espoo, Finland). Serum insulin level was determined using a radioimmunoassay kit (Novo Biolabs; Novo Nordisk, Bagsvaerd, Denmark). The serum samples were stored frozen at −80 °C for 0.2–2.5 years. The between batch coefficient of variation was 8.9 % for 65 pmol/L and 17.5 % at 222 pmol/L (n = 10). The values obtained were immunoreactive insulin as the assay has cross-reactivity with proinsulin. A glucose dehydrogenase method (Merck, Darmstadt, Germany) was used to assess the blood glucose after precipitation of proteins by trichloroacetic acid. Insulin resistance was estimated as follows: HOMA-IR = fasting plasma insulin (µU/mL) × FPG (mmol/L)/22.5. The cholesterol contents of lipoprotein fractions and serum triglycerides were measured enzymatically (Boehringer Mannheim, Mannheim Germany), on the day after the high-density lipoprotein (HDL) was separated from fresh samples by ultracentrifugation and precipitation. Low-density lipoprotein (LDL) was estimated as total cholesterol-HDL-triglycerides/5. Serum C-reactive protein (CRP) was measured with an immunometric assay (Immulite High Sensitivity C-Reactive Protein Assay; DPC, Los Angeles, CA).
The resting systolic blood pressure (SBP) was measured between 8 and 10 a.m. with a random-zero sphygmomanometer (Hawksley, Lancing England) by two trained nurses using the following protocol: after supine rest of 5 min, three measurements in supine, one in standing and two in sitting position with 5-min intervals. The SBP was taken as the mean of all 6 measurements. Baseline diseases, smoking habits, years of education, family history of T2DM (defined as positive if a first-degree relative of the subject had diabetes history) and drug therapy were assessed by self-administered questionnaires. The diagnosis of chronic diseases was checked during a medical examination by the internist. Alcohol consumption was assessed using the Nordic Alcohol Consumption Inventory [8]. Detailed descriptions on physical activity estimation have been published previously [8]. BMI was computed as the ratio of weight in kilograms to the square of height in meters; waist circumference was calculated as the average of 2 measurements taken after inspiration and expiration at the midpoint between the lowest rib and iliac crest. Waist-to-hip ratio (WHR) was defined as waist girth/hip circumference measured at the trochanter major [9].
Statistical analysis
For all the analyses, natural logarithm (loge) transformed values of nonnormal distributed variables were used. Descriptive data are presented as means and standard deviation (SD) for continuous variables and percentages for categorical ones; their differences were estimated with ANOVA and chi-square test, respectively. Correlation coefficients were calculated to assess the correlation between fructosamine levels and other continuous variables, whereas mean differences between groups were calculated for categorical factors.
Analyses of the associations between fructosamine and incident T2DM involved Cox-regression modeling. The proportional hazards assumption was verified for all variables by inspection of the plots of the Schoenfeld residual for covariates. Shapes of association were assessed by plotting hazard ratios (HRs) estimated within quartiles of fructosamine relative to the bottom quartile against the mean fructosamine level in each quartile. 95 % confidence intervals (CIs) were estimated from variances attributed to the groups to reflect the amount of information within each group (including the reference category [10]). To assess the independence of association between fructosamine and incident cases of T2DM, we calculated HRs comparing top versus bottom quartile of baseline fructosamine levels with progressive adjustment for potential confounders selected on the basis of their previously established role as predictive factors. In model 1, we included age, BMI, SBP, serum HDL-cholesterol and family history T2DM; in model 2, we added CRP, physical activity and serum triglyceride concentrations. Model 3a included variables in model 2 plus HOMA-IR, while model 3b variables in model 2 plus FPG. Since associations were nearly log-linear (except at low values of fructosamine), we calculated HRs associated with 1-SD higher baseline value of fructosamine.
To enable a comparison with a previous similar analysis in a different population [4], we also estimated associations between fructosamine and incident T2DM with other adjustment models (model A: age, BMI; model B: model A plus LDL and HDL-cholesterol, triglycerides, WHR, SBP, antihypertensive drugs, family history of diabetes mellitus, education level, alcohol use, smoking status and physical activity; model C: model B plus FPG) and for three defined categories of fructosamine levels: <241 µmol/L; 241–264 µmol/L; >264 µmol/L.
All analyses were performed with Stata 12 (Stata Corp, College Station, TX, USA), and two-sided p value <0.05 was considered statistically significant.
Results
Baseline characteristics and numbers of outcome events during the follow-up
At baseline, 33 % were smokers, mean age was 53 years (SD 5 years) and mean fructosamine level was 267 µmol/L (SD 36 µmol/L); fructosamine levels were weakly correlated with other risk factors. Baseline characteristics of the study population and fructosamine correlation coefficients are reported in Table 1.
Subjects in the higher quartiles of fructosamine levels were older, with higher BMI, SBP, LDL- and HDL-cholesterol, FPG and insulin resistance, but smoked less and had lower levels of CRP and triglycerides (Supplementary Table S1). Levels of cardiometabolic risk factors (i.e., BMI, WHR, triglycerides, FPG, HOMA-IR) resulted higher in subjects who had developed T2DM during the follow-up as compared to subjects who had not (Supplementary Table S2).
During a median follow-up time to incident T2DM or the end of follow-up of 23 years (interquartile range: 18–25 years), a total of 153 (6.1 %) new cases of T2DM were recorded, with an unadjusted incidence rate of 2.95 (95 % CI 2.51–3.45) per 1,000 person-years.
Fructosamine as risk factor for incident type 2 diabetes mellitus
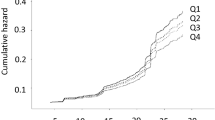
The relationship between baseline fructosamine levels and incident T2DM, progressively adjusted for potential confounders, is depicted in Fig. 1 and Supplementary Figure S1.
Hazard ratios (HRs) and 95 % CI of type 2 diabetes mellitus by quartiles of baseline fructosamine levels. Legend: variables included in the models. Model 1: Age, body mass index, systolic blood pressure, serum HDL-cholesterol and family history type 2 diabetes mellitus; Model 2: Model 1 plus C-reactive protein, physical activity and serum triglyceride; Model 3a: Model 2 plus HOMA-IR; Model 3b: Model 2 plus fasting plasma glucose
In analyses adjusted for age, BMI, SBP, serum HDL-cholesterol and family history T2DM (model 1), we recorded an approximately “J-shaped” associations between fructosamine levels and incident T2DM, with a nadir at about 250 µmol/L; similar associations were found after further adjustment for CRP, physical activity, serum triglyceride (model 2) and HOMA-IR (model 3a). Conversely, no clear relationship was evident when FPG was added to model 2 (Fig. 1 and Supplementary Figure S1; Supplementary Table S3).
To account for nonlinear associations at low fructosamine values, we estimated HRs for 1-SD higher baseline values of fructosamine after the exclusion of 804 participants with fructosamine levels <250 µmol/L, leaving 1,716 participants and 105 cases for the analyses. The HRs for 1-SD (28 µmol/L) higher fructosamine levels were as follows: 1.20 (95 % CI 1.00–1.44; p = 0.046) after adjustment for the variables included in model 1; 1.21 (1.01–1.46; p = 0.041) upon further adjustment for CRP, physical activity and serum triglyceride (model 2); 1.22 (1.01–1.47; p = 0.034) in model 3a, with the further inclusion of HOMA-IR; and 1.05 (0.86–1.27; p = 0.632) in model 3b, with FPG instead of HOMA-IR.
Conclusions
Our results indicate that, in this cohort of middle-age Finnish men, fructosamine is nonlinearly associated with the risk of incident T2DM, with a nadir around 250 µmol/L and a ~20 % higher risk of T2DM for ~30 µmol/L higher baseline levels of fructosamine over this threshold. Such association resulted significant after adjustment for several T2DM risk factors, but was not independent from FPG.
T2DM is a major cause of morbidity and mortality in developed and developing world [11]. Although much progress has been made in the definition and treatment of T2DM risk factors, there is a growing interest in the identification of new biomarkers to help identify subjects at risk of developing T2DM. Common glycemic measures, such as FPG, postload glucose and HbA1c, have been demonstrated to be positively and strongly associated with the risk of T2DM [12–15]. In recent years, however, research has also focused on the predictive role of “alternative” glycemic markers, such as fructosamine. Fructosamine is commonly used in clinical practice to monitor short-term glucose control, whereas HbA1c measurements can be affected by nonphysiological hemoglobin turnovers [16]. In population-based study, fructosamine has been associated with microvascular conditions (particularly retinopathy and chronic kidney disease) [4, 17], while less clear is its association with macrovascular complications in the general population [18, 19]. Moreover, in a small study, fructosamine has been also related to the risk of incident T2DM [3], and such association has been recently confirmed in a larger cohort analysis from the same population [4]. Both analyses have suggested an approximately linear association between baseline fructosamine levels and risk of incident T2DM, while our findings would indicate a “J-shaped” association, with a nadir at approximately 250 µmol/L. Furthermore, in the two previous studies [3, 4], the association was independent from FPG, while our results suggest that fructosamine might not add information regarding T2DM risk independent of FPG.
Several reasons could explain these divergences. This may be due to a true difference, owing to the inherent dissimilar characteristics of the study populations in terms of age, sex, race or genetic background (indeed, differences in glycation processes have been reported according to single nucleotide polymorphisms [5]); or related to the ascertainment of outcomes; or associated with statistical planning and analysis; or a combination of all these. To ease a comparison, however, we have also analyzed our data adjusting for the same covariates and using the same thresholds of fructosamine baseline levels of the previous larger ARIC study [4], still finding a different association with risk of T2DM (Supplementary Figure S2).
Interestingly, “J-shaped” associations have been previously reported for other T2DM risk factors [20]. It should be noted that lower concentrations of serum fructosamine could be related to clinical conditions that are also potentially associated with an increased risk of T2DM (i.e., liver diseases) [2]. In this view, lower fructosamine levels could be a marker of other subclinical diseases and explain the inverse association between its low levels and risk of T2DM. Indeed, although the low number of participants and cases with baseline levels of fructosamine <250 µmol/L limited a formal statistical analysis, subjects with low levels of fructosamine seem to have a significantly higher risk of T2DM even after adjustment for FPG (Supplementary Figure S1); these findings further support the role of low fructosamine as marker of other conditions at increased risk of T2DM. However, additional studies are needed to clarify the association between low fructosamine levels and risk of T2DM.
We should acknowledge some limitations and strengths of this study. First, a generalization of our findings is limited by the study population, consisting of middle-age Finnish men only. As to date data are available only from studies enrolling US participants, these findings need to be confirmed in other ethnic groups. Second, we could not correct for a possible regression dilution bias [21], as we had only baseline assessment of fructosamine values. However, for covariates with available repeated measurements, we observed a rather stable “phenotype” (Supplementary Table S4). Third, only fructosamine-derived HbA1c was available; therefore, we could not evaluate whether fructosamine measurement might provide additional information above that of HbA1c on the risk of T2DM. On the other hand, strengths of this study include the rigorous measurement of baseline risk factors and the assessment of T2DM, the large and homogeneous community-based sample and the long-term follow-up.
In conclusion, in our cohort of middle-age Finnish men, baseline fructosamine levels were associated with T2DM risk in a “J-shaped” fashion, with the lowest risk at 250 µmol/L; over this threshold, the risk was mainly explained by parallel higher levels of FPG. Further studies are necessary to assess the shape of association in other populations and to clarify the meaning of low fructosamine levels as risk factor of T2DM.
References
Herder C, Kowall B, Tabak AG, Rathmann W (2014) The potential of novel biomarkers to improve risk prediction of type 2 diabetes. Diabetologia 57(1):16–29
Armbruster DA (1987) Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 33:2153–2163
Juraschek SP, Steffes MW, Miller ER 3rd, Selvin E (2012) Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care 35(11):2265–2270
Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J (2014) Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2(4):279–288
Tanhäuserová V, Kuricová K, Pácal L, Bartáková V, Rehořová J, Svojanovský J, Olšovský J, Bělobrádková J, Kaňková K (2014) Genetic variability in enzymes of metabolic pathways conferring protection against non-enzymatic glycation versus diabetes-related morbidity and mortality. Clin Chem Lab Med 52(1):77–83
http://www.strobe-statement.org/. Accessed 23 April 2014
Salonen JT (1988) Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res 20:46–50
Lakka TA, Venäläinen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT (1994) Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med 330:1549–1554
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288(21):2709–2716
Easton DF, Peto J, Babiker AG (1991) Floating absolute risk: an alternative to relative risk in survival and case–control analysis avoiding an arbitrary reference group. Stat Med 10:1025–1035
http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf. Accessed 23 April 2014
Rathmann W, Kowall B, Heier M, Herder C, Holle R, Thorand B, Strassburger K, Peters A, Wichmann HE, Giani G, Meisinger C (2010) Prediction models for incident type 2 diabetes mellitus in the older population: KORA S4/F4 cohort study. Diabet Med 27(10):1116–1123
Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, Webb D (2013) Progression rates from HbA1c 6.0–6.4 % and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 56(7):1489–1493
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A, Israeli Diabetes Research Group (2005) Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353(14):1454–1462
Kolberg JA, Jørgensen T, Gerwien RW, Hamren S, McKenna MP, Moler E, Rowe MW, Urdea MS, Xu XM, Hansen T, Pedersen O, Borch-Johnsen K (2009) Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care 32(7):1207–1212
Saudek CD, Derr RL, Kalyani RR (2006) Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 295:1688–1697
Selvin E, Francis LM, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, Steffes MW (2011) Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 34(4):960–967
Browner WS, Pressman AR, Lui LY, Cummings SR (1999) Association between serum fructosamine and mortality in elderly women: the study of osteoporotic fractures. Am J Epidemiol 149(5):471–475
Shafi T, Sozio SM, Plantinga LC, Jaar BG, Kim ET, Parekh RS, Steffes MW, Powe NR, Coresh J, Selvin E (2013) Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care 36(6):1522–1533
Sakurai M, Miura K, Takamura T, Ishizaki M, Morikawa Y, Nakamura K, Yoshita K, Kido T, Naruse Y, Kaneko S, Nakagawa H (2009) J-shaped relationship between waist circumference and subsequent risk for Type 2 diabetes: an 8-year follow-up of relatively lean Japanese individuals. Diabet Med 26(8):753–759
Hughes MD (1993) Regression dilution in the proportional hazards model. Biometrics 49:1056–1066
Acknowledgments
We thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health, and University of Eastern Finland, Kuopio, Finland, for data collection in the study.
Conflict of interest
Francesco Zaccardi, Sudhir Kurl, Dario Pitocco, Kimmo Ronkainen and Jari A. Laukkanen declare that they have no conflict of interest.
Ethical statement
The study was approved by the Research Ethics Committee of the University of Eastern Finland.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Porta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zaccardi, F., Kurl, S., Pitocco, D. et al. Serum fructosamine and risk of type 2 diabetes mellitus among middle-age Finnish men: a 23-year population-based prospective study. Acta Diabetol 52, 161–166 (2015). https://doi.org/10.1007/s00592-014-0625-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0625-8