Abstract
A state of subclinical systemic inflammation is characteristically present in obesity/insulin resistance and type 2 diabetes mellitus (T2DM). The aim of the study was to develop an integrated measure of the circulating cytokines involved in the subclinical systemic inflammation and evaluate its relation with whole-body insulin sensitivity and glucose metabolism in T2DM. T2DM patients (n = 17, M/F 13/4, age = 55.0 ± 1.7 years, BMI = 33.5 ± 1.5 kg/m2, HbA1c = 7.7 ± 0.3 %) and normal glucose-tolerant (NGT) subjects (n = 15, M/F 7/8, age = 49.1 ± 2.5 years, BMI = 31.8 ± 1.2 kg/m2, HbA1c = 5.6 ± 0.1 %) were studied in a cross-sectional design. Whole-body insulin sensitivity was quantified by the euglycemic clamp. Beta-cell function [disposition index (DI)] was calculated using insulin and glucose values derived from an oral glucose tolerance test and the euglycemic clamp. Body fat mass was evaluated by dual-energy X-ray absorptiometry. Plasma cytokine [TNF-α, IL-6, MCP-1, osteopontin, fractalkine and adiponectin] values were divided into quintiles. A score ranging from 0 (lowest quintile) to 4 (highest quintile) was assigned. The inflammatory score (IS) was the sum of each cytokine score from which adiponectin score was subtracted in each study subject. Inflammatory cytokine levels were all higher in T2DM. IS was higher in T2DM as compared to NGT (10.0 ± 1.1 vs. 4.8 ± 0.8; p < 0.001). IS positively correlated with fasting plasma glucose (r = 0.638, p < 0.001), 1-h plasma glucose (r = 0.483, p = 0.005), 2-h plasma glucose (r = 0.611, p < 0.001) and HbA1c (r = 0.469, p = 0.007). IS was inversely correlated with insulin sensitivity (r = −0.478, p = 0.006) and DI (r = −0.523, p = 0.002). IS did not correlate with BMI and body fat mass. IS was an independent predictor of fasting plasma glucose and had a high sensibility and sensitivity to predict insulin resistance (M/I < 4). A state of subclinical inflammation defined and quantifiable by inflammatory score including TNF-α, IL-6, MCP-1, osteopontin, fractalkine and adiponectin is associated with both hyperglycemia and whole-body insulin resistance in T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance, impaired insulin secretion and hyperglucagonemia are important determinants in the natural history and pathogenesis of hyperglycemia in type 2 diabetes mellitus (T2DM) [1, 2]. A number of studies have demonstrated that subclinical inflammation likely plays an important role in the pathogenesis of insulin resistance and T2DM. Moreover, it is well established that obesity is associated with a state of chronic low-grade inflammation, characterized by alterations in circulating immune-modulatory factors and adipose tissue resident immune cells which may provide a causal link between increased adiposity and IR [3]. Adipose tissue is in fact an endocrine organ, secreting a variety of cytokines (TNF-α, IL-6, IL-1β) predominantly produced by local macrophages that could comprise up to 40 % of total cells in obese adipose tissue, as well as leptin, which has also been implicated in mediating cardiac hypertrophy in human obesity [4–6]. MCP1 (also known as CCL2), which signals to macrophages through the CCR2 receptor, is strongly correlated with obesity [7–9]. Osteopontin (OPN), a cytokine secreted by the adipose tissue, binds with integrins and CD44 receptors to recruit macrophages and T cells to inflammatory sites [10–12]. Fractalkine (FRK) is expressed in several cell types such as macrophages, endothelial cells and adipocytes. FRK may contribute to atherogenesis by affecting cell migration, adhesion and proliferation [13]. Adiponectin (APN), mostly secreted by adipocytes, has potent anti-inflammatory effects, likely by activating ceramidase, that reduce intracellular levels of pro-inflammatory ceramides, while increasing the concentration of sphingosine-1-phosphate, a molecule with immunoregulatory and anti-inflammatory effects [14]. The subclinical systemic inflammation of T2DM is also associated with islet inflammation. In fact, islets from patients with T2DM display typical features of tissue inflammation that contributes to beta-cell dysfunction [15–17].
The aim of this study was to evaluate whether an integrated measure of circulating inflammatory markers, which we called inflammatory score (IS), could improve estimation of the relation between whole-body insulin sensitivity, subclinical systemic inflammation and hyperglycemia in T2DM.
Materials and methods
Study design
Seventeen patients with T2DM treated with diet alone or diet plus metformin and/or sulfonylurea and fifteen subjects with normal glucose tolerance (NGT) participated in the study.
The diabetic patients were free of other major organ disease, had a stable body weight for at least 3 months and had not participated in strenuous exercise prior to enrollment.
Of the 17 T2DM patients, three were drug naïve, while the others were treated with metformin (n = 8) or a combination of metformin and sulfonylurea (n = 6).
The control subjects had NGT according to ADA criteria, were free of other major organ disease and had a stable body weight for at least 3 months. All subjects underwent OGTT, euglycemic–hyperinsulinemic clamp and determination of plasma adipocytokines and inflammatory markers (FRK, TNF-α, IL-6, MCP-1, OPN and APN).
Ethics
The study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center and of the South Texas Veterans Healthcare System, Audie Murphy Hospital at San Antonio, Texas. The study protocol was conducted in accordance with the guidelines of the Declaration of Helsinki. Written and oral informed consent was obtained from all participants enrolled in this study.
Study procedures
All metabolic studies were carried out in the morning at The Bartter Clinical Research Unit of the South Texas Veterans Healthcare System, following a 10–12-h overnight fast.
-
I.
OGTT A catheter was placed in an antecubital vein, and blood samples were collected at −30, −15, 0, 30, 60, 90 and 120 min for the determination of plasma glucose (PG), C-peptide and insulin concentrations.
-
II.
Insulin secretion/IR index (also called disposition index) was calculated as [ΔISR 0–120 (AUC)/ΔG 0–120 (AUC)] × [glucose infusion rate (M)/steady-state plasma insulin concentration (I)].
-
III.
Euglycemic–hyperinsulinemic clamp After an overnight fast, catheters were inserted into an antecubital vein for insulin and glucose infusion, and the second catheter was inserted retrogradely into a vein on the dorsum of the hand, which was placed into a thermoregulated heated box (55 °C). Following collection of three baseline samples, participants received a 4-h euglycemic insulin clamp (80 mU m−2 min). The PG concentration was allowed to drop to 5.6 mmol/l, at which it was maintained. Under steady-state conditions of euglycemia, the glucose infusion rate (M) divided by the steady-state plasma insulin concentration (I) provides a direct measure of whole-body insulin sensitivity (M/I value; μmol kg−1 min−1 (pmol/l)−1].
-
IV.
DEXA DEXA was performed to determine fat and lean body mass and bone mineral density (Hologic, Waltham, MA, USA).
-
V.
Inflammatory score The inflammatory score was calculated as follows. Each plasma cytokine value was stratified into quintiles to determine cutoff points and assign a score ranging from 0, which was assigned to the lowest quintile, to 4, which was assigned to the highest quintiles. FRK, TNF-α, MCP-1, IL-6 and OPN are pro-inflammatory cytokines, while APN is an anti-inflammatory adipocytokine. Therefore, the inflammatory score was calculated for each study subject as the sum of each cytokine score derived from the pro-inflammatory cytokines from which the score derived from the APN was subtracted.
-
VI.
Adipose Tissue Insulin Resistance Index (ATIRI) was the product of fasting plasma free fatty acid (FFA) and fasting plasma insulin levels.
Biochemical analyses
Serum concentrations of circulating cytokines (TNF-α, IL-6, MCP-1, FRK and OPN) were measured using the human-specific Milliplex map kit according to the manufacturer’s instructions (Millipore, St Charles, MO, USA). Total APN was measured by ELISA (R&D Systems, Minneapolis, MN, USA). PG levels were measured using the glucose oxidase method (GM9; Analox Instruments, London, UK). Plasma insulin and C-peptide were measured by RIA (Siemens Medical Solutions Diagnostics, Tarrytown, NY).
Statistical analysis
Values were calculated as mean ± SEM or as median (interquartile range) for variables with a skewed distribution. Variables that were not normally distributed were log-transformed before analysis. A p < 0.05 (two-tailed analysis) was considered to be statistically significant. The Mann–Whitney test was used to test differences in anthropometric and metabolic parameter between the two study groups. Treatment-induced changes were compared using Wilcoxon’s signed-rank test. Pearson correlation coefficients were used to assess the strength of the relationship between the variables studied. Multivariate analysis was also performed to evaluate the contribution of the inflammatory score and cytokines to the prediction of insulin resistance and fasting plasma glucose levels. To quantify the predictive value of cytokines and inflammatory score, we constructed receiver-operating-characteristic (ROC) curves and determined the area under the curve (AUC). The outcome variable was M/I < 4, which was considered as insulin resistance. Data were analyzed using SPSS 20 (Statistical Package for Social Sciences, Chicago, IL, USA).
Results
Clinical characteristics of the study population
Type 2 diabetes mellitus patients were well matched in terms of age, gender, BMI, waist circumference, fat mass and blood pressure with the NGT subjects. However, T2DM patients had a more favorable lipid profile because of the lipid-lowering treatment (13 were treated with statins: 8 with simvastatin and 5 with rosuvastatin, while 4 with diet alone), except for higher triglycerides levels, as compared with NGT. T2DM patients had higher plasma glucose levels, HbA1c, insulin and c-peptide levels associated with lower whole-body insulin sensitivity, beta-cell function and insulin secretion. Also, T2DM patients had higher fasting plasma FFA levels and higher ATIRI as compared with NGT (Table 1).
Inflammatory cytokines and inflammation score
All cytokine plasma levels were increased in T2DM patients, and in particular plasma OPN and MPC-1 levels were one to two fold higher in T2DM patients compared with NGT subjects. Plasma adiponectin levels were significantly higher in NGT compared with T2DM group. The inflammatory score, which includes pro-inflammatory and anti-inflammatory cytokine values, was significantly higher in T2DM patients than in NGT subjects (10.0 ± 1.1 vs. 4.8 ± 0.8; p < 0.001) (Fig. 1). The inflammatory score for each cytokine was consistently higher in T2DM group compared to NGT (TNF-α 2.5 ± 0.3 vs. 1.5 ± 0.3 pg/ml; p = 0.04; IL-6 2.1 ± 0.4 vs. 1.7 ± 0.4 pg/ml; p = 0.4; FRK 2.1 ± 0.3 vs. 1.8 ± 0.5 pg/ml; p = 0.6; OPN 6802.1 ± 1371.1 vs. 2467.3.4 ± 423.6 ng/ml; p < 0.001; MCP-1 2.9 ± 0.3 vs. 1.1 ± 0.2 pg/ml; p < 0.001; APN 7125.5 ± 992.9 vs. 4369.4 ± 438.2 ng/ml; p = 0.001, respectively) (Supplementary Figure 1).
The BMI ranged from 22.2 to 44.3 kg/m2 in all study population. Interestingly, the inflammatory score values and the plasma cytokine levels were not related to BMI, as demonstrated by the univariate analysis between BMI, all cytokines and the inflammatory scores (Supplementary Table 1). There were statistically significant positive correlations between these cytokines. Interestingly, OPN correlated with FRK, TNF-α, MCP-1 and IL-6 (r = 0.597, 0.659, 0.640, 0.487, all p < 0.001), while it did not correlate with APN (r = −0.236, p = 0.193). In a stepwise regression analysis, MCP-1 and OPN were the strongest independent predictors of TNF-α (adjusted R 2 = 0.49, p < 0.001) (Supplementary Table 2a), while TNF-α was the strongest predictor of MCP-1 (adjusted R 2 = 0.49; p < 0.001) (Supplementary Table 2b).
Inflammatory score and glucose metabolism
The inflammatory score was strongly and positively correlated in the entire sample with fasting plasma glucose (r = 0.638, p < 0.001) (Fig. 2a), 1-h plasma glucose (r = 0.483, p = 0.005) (Fig. 2b), 2-h plasma glucose (r = 0.611, p < 0.001) (Fig. 2c) and the HbA1c (r = 0.469, p = 0.007) (Fig. 2d). The pro-inflammatory cytokines were positively and significantly correlated with fasting plasma glucose, although the correlation did not reach the statistical significance for FRK and IL-6 (Table 2 and Supplementary Figure 2). APN was inversely correlated with the fasting plasma glucose, although this was not statistically significant (data not shown).
The multivariate analyses were performed on the entire population (Model 1, Table 2). All cytokines, except for FRK, were independent predictors of fasting plasma glucose. Moreover, in the multivariate analysis including either cytokine levels as described in Model 1 or the inflammatory score as described in Model 2 (Table 2), the inflammatory score remained a strong independent predictor of fasting plasma glucose variance (adjusted R 2 = 0.32; p = 0.01), improving the capability to predict the fasting plasma glucose of any single inflammatory cytokines considered in the study.
Similarly, the inflammatory score was significantly inversely correlated with the whole-body insulin sensitivity (r = −0.478, p = 0.006) (Fig. 3a) and disposition index (r = −0.523, p = 0.002) (Fig. 3b).
In bivariate analyses where insulin sensitivity (M/I) was the dependent variable, plasma TNF-α (r = −0.40; p = 0.023), MCP-1 (r = −0.51; p = 0.003) and OPN (r = −0.43; p = 0.01) values were strongly inversely correlated with the M/I values (Table 3 and Supplementary Figure 2). In the multivariate analysis, including all cytokines as independent variables, MCP-1 was a strong independent predictor of insulin sensitivity (adjusted R 2 = 0.18; p = 0.03) (Model 1, Table 3). The inflammatory score did not improve the prediction of the insulin sensitivity variance as compared to Model 1 (Model 2, Table 3).
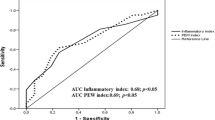
The receiver-operating characteristic analysis demonstrated that it was possible to differentiate between insulin-resistant and insulin-sensitive subjects where insulin resistance was defined as M/I < 4 (Supplementary Figure 3). AUC ROC values for determining insulin resistance were computed for the inflammatory score, plasma cytokine values and metabolic parameters. The AUC ROC for MCP-1, TNF-α, the inflammatory score and fasting plasma glucose were 0.79, 0.71, 0.75 and 0.79, respectively (all p < 0.05).
ATIRI was strongly correlated with FRK (r = 0.411; p = 0.02), MCP-1 (r = 0.497; p < 0.01), OPN (r = 0.495; p = 0.005) and inflammatory score (r = 0.375; p = 0.03), while it did not correlate with TNF-α, IL-6 and APN (Supplementary Table 3a). In the multivariate analysis, including all cytokines as independent variables, FRK, TNF-α, MCP-1, IL-6, OPN and APN were all independent predictors of ATIRI (Model 1, Supplementary Table 2b). The inflammatory score did not improve the prediction of the adipose tissue insulin resistance variance as compared to Model 1 (Model 2, Supplementary Table 3b).
Discussion
The present study demonstrates that the inflammatory score, an integrated measure of subclinical systemic inflammation including four inflammatory (TNF-α, IL-6, MCP-1 and FRK) and an anti-inflammatory cytokine (APN), is increased in T2DM individuals and strongly correlates with whole-body insulin sensitivity (directly evaluated by the euglycemic clamp), β-cell function, glucose levels in the OGTT and HbA1c. The inflammatory score is an independent predictor of fasting plasma glucose variance and also correlates with high sensitivity and specificity with insulin resistance in this population. Inflammatory markers such as high-sensitivity C-reactive protein are independent predictors of coronary heart disease and improve global classification of cardiovascular diseases risk, regardless of the LDL cholesterol level and hyperglycemia [18, 19]. Interestingly, the benefits of metformin on macrovascular complications of diabetes could be partially explained by the inhibition of pro-inflammatory responses through direct inhibition of NF-kB and the PI3 K–Akt pathways, although clinical studies have failed to demonstrate its effect on plasma cytokine concentration in type 2 diabetes [20, 21].
Recent evidences also suggest that glycemic instability/variability may contribute to the development of more severe diabetic complications. In fact, postprandial hyperglycemia is a very frequent phenomenon in patients with T2DM, even when the patients are on active pharmacological treatments [22]. Although the relation between glycemic instability and the risk of cardiovascular disease and diabetic complications is complex, it has been demonstrated that repeated fluctuations of glucose produce increased circulating levels of inflammatory cytokines compared with stable high glucose in normal subjects and worsened endothelial dysfunction in both normal subjects and T2DM patients [23]. Offspring of T2DM patients who had impaired glucose tolerance also have higher levels of inflammatory cytokines, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E-selectin and vascular adhesion protein-1 correlated with inflammatory markers [24, 25]. Interestingly, a decrease in daily glucose excursions with vildagliptin, a DPPIV inhibitor, decreases oxidative stress, inflammatory cytokines and IMT, a surrogate marker for early atherosclerosis in T2DM subjects, possibly mediated by an improvement in vascular inflammation and endothelial dysfunction [26]. The inflammatory score is strongly correlated with components of postprandial glucose (1-h and 2-h plasma glucose) and predicts fasting glucose, emphasizing somehow an intimate connection between hyperglycemia and subclinical inflammation.
A link between low-grade inflammation and obesity/insulin resistance has been demonstrated in several studies [27–29]. TNF-α and other pro-inflammatory cytokines (IL-1 and IL-6, interferon-γ) inhibit insulin-mediated tyrosine phosphorylation of the insulin receptor and insulin receptor substrate (IRS)-1, leading to defective activation of downstream insulin signaling to phosphatidylinositol-3 (PI3)-kinase and translocation of GLUT4 to the cell surface [30]. Interestingly, also MCP-1 correlates strongly with insulin resistance in vivo in humans, consistent with our findings [31].
In humans, a dysregulation of the TIMP3–TACE dyad, which regulates, among other things, the TNF-α shedding from pro-TNF-α, is present in obese individuals with type 2 diabetes [32]. Moreover, T2DM patients exhibit reduction of TIMP-3, as well as increased activity of ADAM17 and MMP9, in atherosclerotic plaques. Therefore, a metabolic-dependent reduction in TIMP3 expression may increase the activity of inflammatory and proteolytic enzymes, which may play a role in atherothrombosis [33]. Of note, low-dose pioglitazone treatment reduced TNF-α expression and also TACE enzymatic activity in human skeletal muscle, and these effects were associated with an improvement in HbA1c, FPG, insulin sensitivity and APN, supporting the notion of a link between inflammation, IR and hyperglycemia, while sulfonylurea treatment failed to demonstrate a reduction in acute phase markers and plasma cytokines concentration in type 2 diabetic patients [34]. The inflammatory score is also associated with ATIRI, an independent surrogate measure of insulin sensitivity that is easily available in the ambulatory setting for large population studies [35].
The association between the inflammatory score and the T2DM abnormalities is also evident in the strong correlation with beta-cell function. A higher inflammatory score is associated with an impaired beta-cell function in keeping with several studies that have shown that the histology of islets from patients with T2DM displays typical features of tissue inflammation, including higher expression of cytokines and chemokines, immune cell infiltration, decreased insulin staining, β-cell apoptosis and islet amyloidosis [2, 17].
Hyperglycemia is sensed by the inflammasomes, innate immune sensors that detect metabolic danger signals. The assembly of the inflammasome activates caspase-1 that cleaves pro-IL-1β into active IL-1β, resulting in the release of a broad array of cytokines, followed by recruitment of immune cells including macrophages and damage of the beta-cells. Hyperglycemia is also critically important to determine oxidative stress in both type 1 and type 2 diabetes and is tightly connected with inflammation possibly by activation on NFkβ [36–38]. Conversely, APN is an antidiabetic/anti-inflammatory adipokine that enhances insulin action by several mechanisms, including suppression of gluconeogenesis and regulation of fatty acid metabolism as well as modulation of calcium signaling in skeletal muscles [39–42].
The inflammatory score was calculated considering the APN anti-inflammatory properties in order to obtain a more comprehensive evaluation of low-grade inflammation. This study suggests that hyperglycemia is also tightly linked to the inflammatory condition in T2DM. Recently, identified cytokines, such as OPN and FRK, could improve the detection of the low-grade inflammation in obese mice and T2DM individuals and also the prediction of glucose metabolism abnormalities ([43] and this study).
A strength of this study is the direct comparison of these inflammatory markers between NGT and T2DM subjects, and their relation with a direct measure of insulin sensitivity, obtained with the euglycemic clamp. It is among the first studies to characterize and examine these new inflammatory markers in T2DM. The study also has some limitations. First, the sample size is not large, and therefore, it is somehow difficult to adequately evaluate the ability of the inflammatory score to enhance prediction of insulin resistance relative to the individual inflammatory markers. Second, the relation between inflammatory score and glucose metabolism abnormalities in both groups, NGT and T2DM, was evaluated in a cross-sectional study. Future studies need to evaluate the value of the inflammatory score in larger groups of diabetic and nondiabetic individuals who are more representative of the population at large. It is also important that the sensitivity of the inflammatory score to assess changes in insulin resistance and possibly morbidity and mortality risks is established in a randomized clinical trial employing a targeted pharmacological treatment approach, designed to reduce insulin resistance, improve beta-cell function and reduce cardiovascular mortality in T2DM.
In conclusion, this study suggests that the inflammatory score could represent a new tool for the evaluation of the severity of low-grade inflammation in order to identify T2DM patients, possibly at higher risk, because of more severe insulin resistance and beta-cell dysfunction, who need to be treated more aggressively [44].
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- IS:
-
Inflammatory score
- DEXA:
-
Dual-energy X-ray absorptiometry
- FPG:
-
Fasting plasma glucose
- OGTT:
-
Oral Glucose Tolerance Test
- PG:
-
Plasma glucose
- 1 h PG:
-
1-Hour plasma glucose
- 2 h PG:
-
2-Hour plasma glucose
- FRK:
-
Fractalkine
- MCP-1:
-
Monocyte chemoattractant protein-1
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1β
- OPN:
-
Osteopontin
- IR:
-
Insulin resistance
References
Bonadonna RC, Del Prato S, Saccomani MP, Bonora E, Gulli G, Ferrannini E, Bier D, Cobelli C, DeFronzo RA (1993) Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest 92(1):486–494
Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, Tene-Perez CE, Goldschmidt L, Hart J, Perego C, Comuzzie AG, Tejero ME, Finzi G, Placidi C, La Rosa S, Capella C, Halff G, Gastaldelli A, DeFronzo RA, Folli F (2009) Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci USA 106(33):13992–13997
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity related insulin resistance. J Clin Invest 112(12):1821–1830
Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, Barbieri M, Morabito A, Paolisso G, Folli F, Pontiroli AE (2005) Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab 90(7):4087–4093
Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wåhlén K, Andersson J, Nordström EA, Blomqvist L, Sjögren A, Forsgren M, Attersand A, Arner P (2005) A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab 90(10):5834–5840
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116(6):1494–1505
Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R (2006) Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30(9):1347–1355
Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, Bruemmer D (2007) Osteopontin mediates obesity induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117:2877–2888
Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-Järvinen H, Oresic M, Kaprio J, Peltonen L (2008) Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med 5:e51
Singh M, Ananthula S, Milhorn DM, Krishnaswamy G, Singh K (2007) Osteopontin: a novel inflammatory mediator of cardiovascular disease. Front Biosci 12:214–221
Cefalu WT (2011) Fractalkine: a cellular link between adipose tissue inflammation and vascular pathologies. Diabetes 60(5):1380–1382
Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE (2011) Receptor mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63
Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY (2002) Glucose-induced beta-cell production of interleukin-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860
Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56:2356–2370
Velloso LA, Eizirik DL, Cnop M (2013) Type 2 diabetes mellitus an autoimmune disease? Nat Rev Endocrinol. doi:10.1038/nrendo.2013.131
Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB (2004) Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351(25):2599–2610
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359(21):2195–2207
Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P (2006) Metformin inhibits proinflammatory responses and nuclear factor κß in human vascular wall cells. Arterioscler Thromb Vasc Biol 26:611–617
De Jager J, Kooy A, Lehert P, Bets D, Wulffelé MG, Teerlink T, Scheffer PG, Schalkwijk CG, Donker AJ, Stehouwer CD (2004) Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 256:1–14
Bonora E, Corrao G, Bagnardi V, Ceriello A, Comaschi M, Montanari P, Meigs JB (2006) Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 49(5):846–854
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072
Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamäki J, Punnonen K, Kainulainen S, Laakso M (2006) Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care 29(12):2714–2720
Ruotsalainen E, Vauhkonen I, Salmenniemi U, Pihlajamäki J, Punnonen K, Kainulainen S, Jalkanen S, Salmi M, Laakso M (2008) Markers of endothelial dysfunction and low-grade inflammation are associated in the offspring of type 2 diabetic subjects. Atherosclerosis 197(1):271–277
Barbieri M, Rizzo MR, Marfella R, Boccardi V, Esposito A, Pansini A, Paolisso G (2013) Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 227(2):349–354
Fernandez-Real JM, Ricart W (1999) Insulin resistance and inflammation in an evolutionary perspective: the contribution of cytokine genotype/phenotype to thriftiness. Diabetologia 42:1367–1374
Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM (2008) Increased skeletal muscle tumor necrosis factor alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 57(3):606–613
Ragogna F, Lattuada G, Ruotolo G, Luzi L, Perseghin G (2012) Lack of association of apoE ε4 allele with insulin resistance. Acta Diabetol 49:25–32
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM (1994) Tumor necrosis factor a inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA 91:4854–4858
Monroy A, Kamath S, Chavez AO, Centonze VE, Veerasamy M, Barrentine A, Wewer JJ, Coletta DK, Jenkinson C, Jhingan RM, Smokler D, Reyna S, Musi N, Khokka R, Federici M, Tripathy D, DeFronzo RA, Folli F (2009) Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia 52:2169–2181
Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M (2009) TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58(10):2396–2401
Tripathy D, Daniele G, Fiorentino TV, Perez-Cadena Z, Chavez-Velasquez A, Kamath S, Fanti P, Jenkinson C, Andreozzi F, Federici M, Gastaldelli A, Defronzo RA, Folli F (2013) Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study. Diabetologia 56(10):2153–2163
Yudkin JS, Panahloo A, Stehouwer C, Emeis JJ, Bulmer K, Mohamed-Ali V, Denver AE (2000) The influence of improved glycaemic control with insulin and sulphonylureas on acute phase and endothelial markers in type II diabetic subjects. Diabetologia 43:1099–1106
Fabbrini E, Magkos F, Conte C, Mittendorfer B, Patterson BW, Okunade AL, Klein S (2012) Validation of a novel index to assess insulin resistance of adipose tissue lipolytic activity in obese subjects. J Lipid Res 53(2):321–324
Folli F, Guzzi V, Perego L, Coletta DK, Placidi C, La Rosa S, Capella C, Socci C, Lauro D, Tripathy D, Jenkinson C, Paroni R, Orsenigo E, Cighetti G, Gregorini L, Staudacher C, Secchi A, Bachi A, Brownlee M, Fiorina P (2010) Proteomics reveals novel oxidative and glycolytic mechanisms in type 1 diabetic patients’ skin which are normalized by kidney-pancreas transplantation. PLoS One 5(3):e9923. doi:10.1371/journal.pone.0009923
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11:136–140
Bierhaus A, Nawroth PP (2009) Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 52(11):2251–2263
Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T (2009) Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 382:51–56
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T (2010) Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464:1313–1319
Aouadi M, Tencerova M, Vangala P, Yawe JC, Nicoloro SM, Amano SU, Cohen JL, Czech MP (2013) Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci USA 110(20):8278–8283
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycaemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55(6):1577–1596
Acknowledgments
This work was presented in part in abstract form at the Endocrine Society Meeting, 2013, in San Francisco, CA, USA. G. D. was supported in part by a fellowship from Fo.Ri.SID, Italy.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daniele, G., Guardado Mendoza, R., Winnier, D. et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 51, 123–131 (2014). https://doi.org/10.1007/s00592-013-0543-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-013-0543-1