Abstract
We determined in non-diabetic persons the risk of fasting and non-fasting glucose levels for pre-diabetes, diabetes, and coronary heart disease (CHD), including the roles of serum C-reactive protein (CRP) and HDL cholesterol, and delineated risk profiles of the pre-diabetic states. Over 7¼ years, 2,619 middle-aged Turkish adults free of diabetes and CHD were studied prospectively. Using different serum glucose categories including impaired fasting glucose (IFG, 6.1–6.97 mmol/L) and impaired glucose tolerance (IGT), outcomes were analyzed by Cox regression. IFG was identified at baseline in 112 and IGT in 33 participants. Metabolic syndrome components distinguished individuals with IFG from those with normoglycemia. Participants with IGT tended to differ from adults in normal postprandial glucose categories in regard to high levels of triglycerides, apoA-I, and CRP. Diabetes risk, adjusted for sex, age, waist circumference, CRP, and HDL cholesterol, commenced at a fasting 5.6–6.1 mmol/L threshold, was fourfold at levels 6.1–6.97 mmol/L. Optimal glucose values regarding CHD risk were 5.0–6.1 mmol/L. Fasting and postprandial glucose values were not related to CHD risk in men; IGT alone predicted risk in women (HR 3.74 [1.16;12.0]), independent of age, systolic blood pressure, non-HDL cholesterol, waist circumference, smoking status, and CRP. HDL cholesterol was unrelated to the development of IFG, IGT, and diabetes, while CRP elevation independently predicted the development of diabetes. IGT independently predicts CHD risk, especially in women. HDL dysfunction associated with low-grade inflammation is a co-determinant of pre-diabetic states and their progression to diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the two intermediate states of abnormal glucose regulation, impaired glucose tolerance (IGT) is recognized to have a modestly elevated risk of CVD and CVD mortality [1–3] higher than impaired fasting glucose (IFG) though these outcome rates were similar in the pre-diabetic states in the large elderly non-diabetic cohort of the ARIC study [4]. Women with IGT tend to have a more atherogenic risk profile than men with IGT long before the diagnosis of clinical diabetes [5, 6]. IFG was demonstrated to have a modest impact on the atherosclerotic process as assessed by coronary artery calcification; this impact retained independence of risk factors only in women [7]. In the non-diabetic population, the role of IFG in CVD risk is still controversial [8, 9], although it is an established risk factor of progression to diabetes [10–12]. Still, in subjects with American Diabetes Association (ADA)-defined IFG, the risk of progressing to diabetes needs further evaluation [2].
It was shown in the case–control Western New York Study on 364 non-diabetic adults that women who progressed from normoglycemia to pre-diabetes had significantly higher adjusted values of markers of endothelial dysfunction (soluble ICAM and E-selectin) than men [13]. But, overall, few data are available whether low-grade inflammation is a constituent of one or both pre-diabetic states. Individuals with a fasting glucose of <5.6–6.1 mmol/L and/or a post-load glucose of <7.8 mmol/L are considered to have normal glucose regulation. Evidence exists newly that such people may diverge with respect to risk of coronary heart disease (CHD) depending on response to glucose loading. An atherogenic risk factor profile was observed in a cross-sectional study in 266 offspring of diabetic patients who had normal glucose tolerance and whose 2-h post-load glucose did not return to the fasting level [14].
Paucity of information exists on differences in the determinants between IFG and IGT, especially in diverse ethnicities. Turks are recognized to have a high prevalence of metabolic syndrome [15] and diabetes [16] and, as a general population, were first to disclose dysfunction of serum HDL [16] and several of its apolipoproteins [17], whereby women were involved in a more pronounced manner than men [18]. Hence, examining the determinants of the two intermediate states might well be informative.
In order to detect an abnormality of glucose regulation, a standard oral glucose tolerance test (GTT) is primarily used. Yet, clarifying the question whether categories of blood glucose levels obtained simply routinely 2 h after breakfast may contribute to assessing risk of diabetes or CHD may help the clinician. We tried to evaluate this point, given that an oral GTT is not performed in the surveys of the Turkish Adult Risk Factor Study (TARF).
We aimed in this prospective study to determine in non-diabetic middle-aged Turks (a) the risk of fasting and non-fasting glucose levels for diabetes and CHD (b) the roles of serum C-reactive protein (CRP) and HDL dysfunction in these processes, and (c) to evaluate the risk factor profile of the pre-diabetic states IFG and IGT.
Population and methods
Subjects
The TARF is a longitudinal population-based cohort study on cardiac disease and its risk factors in adults in Turkey carried out biennially in 59 communities in all geographical regions [19]. It involves a random sample of the Turkish adult population, representatively stratified for sex, age, geographical regions, and for rural–urban distribution [19]. Combined measurements of waist circumference and HDL cholesterol have been first made at the follow-up visit in 1997/1998; the latter examination formed the baseline. Participants, 28 years of age or older at baseline, were examined periodically up to the survey 2009/2010. When individuals with prevalent diabetes (n = 181), CHD alone (n = 217), at baseline, no follow-up (n = 269), glucose concentration <2.8 mmol/L (n = 3), a missing paired glucose value in the follow-up (n = 455) were excluded; the remaining 2,619 participants free of diabetes and CHD composed the cohort of the current study. Subjects with IFG and IGT at baseline were excluded from the analyses for incidence of the respective disorders. The survey conformed to the principles embodied in the Declaration of Helsinki and was approved by the Istanbul University Ethics Committee. Individuals of the cohort gave written consent for participation. Data were obtained by history of the past years via a questionnaire, physical examination of the cardiovascular system, sampling of blood, and recording of a resting 12-lead electrocardiogram.
Measurements of risk variables
Blood pressure (BP) was measured using a sphygmomanometer (Erka, Bad Tölz, Germany) after 10 min of rest while seated on both arms, and the mean of two recordings was recorded. Waist circumference was measured with a tape (Roche LI95 63B 00), the subject standing, at the level midway between the lower rib margin and the iliac crest. Body mass index (BMI) was computed as weight divided by height squared (kg/m2). Self-reported cigarette smoking was categorized into never smokers, former smokers (discontinuance of 3 months or more), and current smokers (regularly 1 or more cigarettes daily).
Plasma concentrations of total and HDL cholesterol (>11 h), fasting triglycerides, and glucose were determined at baseline examination by the enzymatic dry chemistry method using a Reflotron apparatus. LDL cholesterol values were computed according to the Friedewald formula. In the final four surveys, the stated parameters, as well as insulin and CRP values, were assayed in a single central laboratory. Blood samples were shipped to Istanbul and stored in deep-freeze at −75°C until analyzed. Concentrations of insulin were determined by the electrochemiluminescence immunoassay ECLIA on Roche Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). Serum concentrations of apo A–I and B, and CRP were measured by the Behring nephelometry (Behring Diagnostics, Marburg, Germany). External quality control was performed with a reference laboratory in a random selection of 5–6% of participants. Data on baseline triglycerides, CRP, and insulin were available in 74, 83, and 56% of participants, respectively.
Definitions and outcomes
Individuals with type-2 diabetes were diagnosed with criteria of the American Diabetes Association (ADA) [20], namely when plasma fasting glucose was ≥7.0 mmol/L (or 2-h postprandial glucose ≥11.1 mmol/L) and/or the current use of diabetes medication. IFG was identified with a fasting level of 6.1–7.0 mmol/L according to the World Health Organization (WHO) definition [21]. In subjects in whom plasma glucose was measured 1½–2½ h after breakfast (27% of the total sample), a glucose concentration 7.8–11.1 mmol/L was defined as IGT and a level 5.6–7.8 mmol/L was analyzed separately. Individuals with abdominal obesity were identified using cutpoints of ≥95 cm in men [22] and ≥88 cm in women, as assessed in the Turkish Adult Risk Factor study. Homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) was calculated in participants who had concomitant fasting insulin and glucose measurements at baseline. HOMA-IR = fasting insulin (μU/ml)* glucose (mmol/l)/22.5 [23]. Biological evidence of functional defectiveness of HDL and apoA-I particles as discerned by follow-up for outcomes of diabetes and/or CHD has been designated as HDL dysfunction [16–18].
Diagnosis of non-fatal CHD was based on the presence of angina pectoris, of a history of myocardial infarction with or without accompanying Minnesota codes of the ECG [24] or on a history of myocardial revascularization. Typical angina and, in women, age >45 years were prerequisite for a diagnosis when angina was isolated. ECG changes of “ischemic type” of greater than minor degree (Codes 1.1–2, 4.1–2, 5.1–2, 7.1) were considered as myocardial infarct sequelae or myocardial ischemia, respectively. Cause of death was assigned in accordance with the information on the mode of death obtained from first-degree relatives and/or local health personnel, considering also pre-existing clinical and laboratory findings elicited during biennial surveys.
Data analysis
Descriptive parameters were shown as mean ± standard deviation (SD), or in percentages. Due to skewed distribution, values derived from log-transformed (geometric) means were used for serum triglycerides, CRP, insulin, and HOMA. T tests and Pearson’s chi-square tests were used to analyze differences between means and proportions of two groups; ANOVA was used to detect differences between means of multiple groups, followed by pairwise comparisons with Tukey HSD tests; pairwise comparisons with Bonferroni adjustments were made to detect significance between groups of estimated means. Participants with fasting glucose measurements (73% of the sample) were analyzed in 3 groups separated by 5 and 6.1 mmol/L limits. The middle category was additionally divided into 5.0–5.6 mmol/L and 5.6–6.1 mmol/L groups to detect whether the outcomes of diabetes or CHD differed but was to be combined if a difference was lacking. Participants with postprandial glucose measurements were analyzed in 3 groups separated by 6.1 and 7.8 mmol/L limits. In predicting outcomes at baseline examination in multivariate analyses, estimates (and 95% confidence intervals) for hazard ratio (HR) were obtained by use of Cox proportional hazards regression analysis in models that controlled for potential confounders. HRs were expressed in terms of 1 SD increment, in the case of the log-transformed CRP in terms of a threefold increment. A value of P < 0.05 on the two-sided test was considered statistically significant. Statistical analyses were performed using SPSS-10 for Windows (SPSS Inc., Chicago, Ill., Nr. 9026510).
Results
At baseline examination, 1,276 men and 1,343 women (51.3%) were available free of diabetes and CHD. Mean age was 47.8 ± 11.8 years, P = 0.5 across genders. MetS prevailed in 40.1% of men and 43.6% of women. Mean follow-up constituted 7.24 years (total 18,970 person-years). CHD newly developed in 331 subjects (17.3 per 1,000 person-years), and incident type-2 diabetes in 261 individuals (13.8 per 1,000 persons-years). IFG existed at baseline in 112 participants (5.9%) while IGT was detected in 33 persons (4.7%). The fasting glucose 5.6–6.1 mmol/L category showed a prevalence of 14% in males and 18% in females.
Baseline characteristics of the non-diabetic participants are displayed by gender in Table 1 and by stratification into fasting and postprandial glucose categories in Table 2. Following parameters did not differ significantly among any glucose categories: total, HDL- and LDL-cholesterol in either sex; in men also apo A–I, apo B, and BMI (P = 0.095). Individuals with IFG, compared with those in the two normoglycemic groups, were approximately 6 years older and had significantly wider waist girths, higher systolic and diastolic BP, and fasting triglycerides. Participants with IGT were only 2 years and not significantly older (P > 0.05) and differed from adults in the two normal postprandial glucose categories in regard to high levels of fasting triglycerides (2.93 mmol/L), CRP (2.64 mg/L), and apo A–I (1.46 g/L) which albeit did not reach statistical significance.
IFG developed subsequently in a total of 243 participants (12.8%) and IGT in 54 subjects (7.6%). Table 3 depicts logistic regression analyses of 6 risk factors for incident IFG and IGT. IFG was examined only among participants with fasting glucose, tested as a continuous independent variable, while the whole sample was used regarding IGT and glucose was tested in categories in order to distinguish potential separate associations of fasting and postprandial subsets. CRP values were not available in 15% of participants. Fasting normal glucose concentrations were predictive of newly developing IFG in both sexes with an RR 2.8. In men, additionally, non-HDL cholesterol predicted IFG. Among individuals who developed IGT, significant variables were male sex, IFG in each gender and, in men, postprandial 5.6–7.8 mmol/L concentrations. Noteworthy is that HDL cholesterol level was not significantly associated with either for IFG or IGT.
Cox regression analysis of relevant risk factors for incident DM is shown in Table 4, the fasting glucose category <5.0 mmol/L serving as referent. In the sex- and age-adjusted model, IGT and IFG were the major determinants in each sex with HRs of over 4. In addition, the postprandial glucose 5.6–7.8 mmol/L significantly predicted and fasting glucose 5.6–6.1 mmol/L tended to predict diabetes in combined genders. In the model additionally comprising waist circumference, HDL cholesterol and CRP level, with the aim of assessing the role of the balance of anti- and pro-inflammatory processes, CRP was—additive to waist circumference—a significant determinant in each sex (HR 1.26 [95% CI 1.16; 1.38]), more strongly in women, and HDL cholesterol was not significantly associated. Attenuation of HR in men with IGT was consistent with partial mediation by CRP and waist girth, but HR was not affected in women with IGT.
Table 5 shows results of a Cox regression analysis for incident CHD developing during follow-up, separately for sexes. Compared with fasting glucose 5.0–6.1 mmol/L, IFG was not significant while IGT significantly predicted CHD only in women with a HR 3.74 (1.16; 12.0), independent of age, systolic blood pressure, non-HDL cholesterol, waist circumference, smoking status, and CRP. In women, HDL cholesterol and current smoking were not, whereas CRP concentrations were significantly predictive of CHD.
Discussion
In this population-based prospective cohort study comprising over 2,600 non-diabetic middle-aged and elderly Turkish adults, we evaluated postprandial glucose values in approximately one-quarter of the sample and used Cox regression analysis over a longer than 7-years’ follow-up. At baseline, WHO-defined IFG was distincy by the components of MetS and being older. In prospective evaluation, IFG was driven, additively to fasting glucose values, not by CRP, but in men by high triglycerides. HDL cholesterol did not appear protective against risk of pre-diabetes or diabetes in either sex. Both IFG and IGT were fourfold independent determinants of type-2 diabetes in both sexes while the postprandial glucose category 5.6–7.8 mmol/L conferred a 1.8-fold diabetes risk. CRP emerged as a significant determinant of diabetes independent of waist girth. Evidence pointed to CRP mediating IGT’s progression to DM only in men as distinct from women in whom these appeared to operate independently and additively. In non-diabetic people, fasting or non-fasting glucose categories did not independently confer CHD risk except for IGT in women. Hence, use of the ADA-defined pre-diabetes is justified among Turks regarding diabetes risk, but the WHO-defined pre-diabetes better indicates threshold with respect to CHD risk.
Pre-diabetes was reported to affect 16.4% of Australian adults [25]. In the pooled DECODE data [6], IFG and IGT prevailed in 15.1 and 14.4%, respectively, of middle-aged and elderly populations (M:F ratio 1.06). In middle-aged Turks, the rate of WHO-defined IFG was 5.9% and ADA-defined IFG was 22.8%, whereas the rate for IGT was 4.7%. Were oral GTT systematically performed in the entire sample, rates of the intermediate states might be estimated to converge toward the DECODE data. Inclusion in our sample of fasting glucose category 5.6–6.1 mmol/L raised the IFG prevalence nearly fourfold, in accordance with the estimate by the ADA Consensus statement [2].
Subjects with isolated IFG in the ARIC study were more likely to smoke and had higher obesity measures and fasting insulin than those with isolated IGT, who had higher triglycerides and white cell counts [4]. Isolated IFG was more common among blacks and white men, and women were more likely to have isolated IGT.
HDL function associated with low-grade inflammation mediates development of IFG and IGT
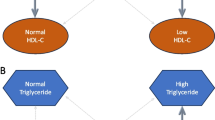
In prospective analysis, IFG was determined—in addition to baseline fasting glucose—in men by atherogenic dyslipidemia as evidenced by HDL- and non-HDL cholesterol significantly predicting it, the latter representing triglyceride-rich (including remnant) lipoproteins. Characteristics in cross-sectional and prospective assessments indicate rather that independent factors in IGT in the present sample were male sex, IFG, a tendency to lack of protection by HDL and apo A–I as marker of a pro-inflammatory state and, in women, age reflecting emergence of enhanced low-grade inflammation with menopause. This suggests that HDL dysfunction among Turks is not concomitant with but precedes by several years the development of diabetes. Turks possessing largely functionally heterogeneous HDL end up with diabetes along two pathways (Fig. 1): either by low HDL (among participants with normally functioning HDL) yielding IFG-mediated diabetes, or by dysfunctional HDL (in relatively high concentrations) leading to diabetes mediated by IGT and low-grade inflammation. Males generally tend to the former and females to the latter pathway.
Schematic representation of factors leading to impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and type-2 diabetes. Hypertriglyceridemia (HTg) induces low HDL cholesterol concentrations which, with or without wide waist girth and elevated blood pressure (BP) lead to IFG in either sex, from whence diabetes develops directly (metabolic syndrome pathway) or via IGT. A second pathway involves enhanced inflammation and HDL dysfunction which lead to IGT, particularly in women, and thence to diabetes
Impact of impaired glucose regulation on CHD
Hyperglycemic clamp studies revealed fasting hyperglycemia was predominantly associated with hepatic insulin resistance and decreased first-phase insulin secretion, whereas an elevated postprandial glucose level was associated with peripheral insulin resistance and impairment of both early- and late-phase insulin release [26].
Inflammation in the prediabetic state was shown in the Insulin Resistance Atherosclerosis Study to be related to increased insulin resistance rather than decreased insulin secretion [27]. Individuals with decreased acute insulin response tended to have lower levels of inflammatory proteins (CRP and PAI-1) compared with those with high insulin secretion, as assessed using a frequently sampled intravenous glucose tolerance test.
Our findings are in agreement with a recent meta-analysis [28] that the CHD risk imparted by IFG is limited and essentially not independent of the associated remaining components of the MetS, hence, presumably related to insulin resistance and genetic factors. In contrast, IGT probably impacts CHD similar to diabetes, namely via the associated systemic inflammation, increased advanced glycation endproducts [29] and the related dysfunction of HDL particles and the apolipoproteins [18, 30]. This extends the notion on the etiology of isolated IGT being predominantly related to physical inactivity, unhealthy diet, and short stature [31]. In non-diabetic Turkish women, an IR-independent enhanced pro-inflammatory state, arising from dysfunctional HDL and high CRP, possibly promoted by post-menopausal decline in sex hormone-binding globulin, seemed to be determinants of IGT, as well as of diabetes [18]. This view is in agreement with the Reynolds Risk Score, an algorithm improved by incorporation of CRP for assessing cardiovascular risk in women [32].
Utility of different glucose criteria in risks for diabetes and CHD
Present findings implicate that categorization by fasting glucose level is appropriate to predict progression to diabetes but is inaccurate—in accord with previous statements [33]—for independently estimating the magnitude of CHD risk that is more appropriately reflected by characteristics of post-load glucose levels. The combination of rare variants of the APOC3 promoter polymorphisms may result in a combination of both disturbance in glucose homeostasis and unfavorable lipoprotein profile, as shown by glucose and fat tolerance tests in the EARS study [34]. A link between postprandial glucose (associated with low-grade inflammation) and triglyceride levels augmenting the risk of CHD (via HDL dysfunction) needs further exploration. Lifestyle implications are that avoiding excess fat and starch in meals is of paramount importance in men, whereas physical activity and avoiding weight gain and obesity are so in women, especially in peri and postmenopausal ages.
Our findings suggest that modestly elevated glucose concentrations (up to 7.8 mmol/L) roughly 2 h after a breakfast implicate by nearly twofold an elevated risk of incident diabetes, an observation that may be of clinical interest because patients often visit the physician in a non-fasting state.
Limitations
Serum glucose measurements were performed in the present study in the fasting state, except 2 h after a breakfast in just over a quarter of the sample; a standard oral GTT was not administered. Thus, a considerable proportion of IGT may not have been disclosed and may rest classified as IFG or normal glycemia. This might have led to underestimation in differences and impact on cardiometabolic risk between the two conditions, though elicited findings are not systemetically biased.
Conclusions
Inclusion of the fasting glucose 5.6–6.1 mmol/L category raised the IFG prevalence nearly fourfold and modestly elevated the risk of progression to diabetes. HDL cholesterol levels were not associated with reduced risk against the development of pre-diabetic states of IFG or IGT as well as of diabetes; and CRP elevation was an additive determinant of IGT’s progression to diabetes. Optimal glucose values regarding CHD risk are 5.0–6.1 mmol/L among Turks. In non-diabetic men, fasting and postprandial glucose values are not independently related to CHD risk, whereas in women CHD risk is significantly and independently elevated in IGT.
References
Festa A, D’Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM (2004) Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 53:1549–1555
Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B (2007) American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759
Zhang L, Qiao Q, Tuomilehto J, Hammar N, Alberti KG, Eliasson M, Heine RJ, Stehouwer CD, Ruotolo G, DECODE Study Group (2008) Blood lipid levels in relation to glucose status in European men and women without a prior history of diabetes: the DECODE study. Diabetes Res Clin Pract 82:364–377
Pankow JS, Kwan DK, Duncan BB, Schmidt MI, Couper DJ, Golden S, Ballantyne CM (2007) Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance: the Atherosclerosis Risk in Communities Study. Diabetes Care 30:325–331
Haffner SM, Miettinen H, Stern MP (1997) Relatively more atherogenic coronary heart disease risk factors in prediabetic women than prediabetic men. Diabetologia 40:711–717
Zhang L, Qiao Q, Tuomilehto J, Hammar N, Ruotolo G, Stehouwer CD, Heine RJ, Eliasson M, Zethelius B, DECODE Study Group. (2009) The impact of dyslipidaemia on cardiovascular mortality in individuals without a prior history of diabetes in the DECODE Study. Atherosclerosis 206:298–302
Moebus S, Stang A, Möhlenkamp S, Dragano N, Schmermund A, Slomiany U, Hoffmann B, Bauer M, Broecker-Preuss M, Mann K, Siegrist J, Erbel R, Jöckel KH, Heinz Nixdorf Recall Study Group (2009) Association of impaired fasting glucose and coronary artery calcification as a marker of subclinical atherosclerosis in a population-based cohort—results of the Heinz Nixdorf Recall study. Diabetologia 52:81–89
Björnholt JV, Erikssen G, Aaser E, Sandvik L, Nitter-Hauge S, Jervell J, Erikssen J, Thaulow E (1999) Fasting blood glucose: an underestimated risk factor for cardiovascular death. Diabetes Care 22:45–49
Hanefeld M, Temelkova-Kurktschiev T, Schaper F, Henkel E, Siegert K, Köhler C (1999) Impaired fasting glucose is not a risk factor for atherosclerosis. Diabet Med 16:212–218
Abdul-Ghani MA, Williams K, DeFronzo R, Stern M (2006) Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 29:1613–1618
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC (2000) The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 23:1108–1112
Stern MP, Williams K, Haffner SM (2002) Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 136:575–581
Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M (2007) Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care 30:354–359
Succurro E, Marini MA, Grembiale A, Lugarà M, Andreozzi F, Sciacqua A, Hribal ML, Lauro R, Perticone F, Sesti G (2009) Differences in cardiovascular risk profile based on relationship between post-load plasma glucose and fasting plasma levels. Diabetes Metab Res Rev 25:351–356
Onat A, Ceyhan K, Başar Ö, Erer B, Toprak S, Sansoy V (2002) Metabolic syndrome: major impact on coronary risk in a population with low cholesterol levels—a prospective and cross-sectional evaluation. Atherosclerosis 165:285–292
Onat A, Can G, Ayhan E, Kaya Z, Hergenç G (2009) Impaired protection against diabetes and coronary disease by high-density lipoproteins in Turks. Metabolism 58:1393–1399
Onat A, Hergenç G, Bulur S, Uğur M, Küçükdurmaz Z, Can G (2010) The paradox of high apolipoprotein A-I levels independently predicting incident type-2 diabetes among Turks. Int J Cardiol 142:72–79
Onat A, Hergenç G (2011) Low-grade inflammation and dysfunction of high-density lipoprotein and its apolipoproteins as a major driver of cardiometabolic risk. Metabolism 60:499–512
Onat A (2001) Risk factors and cardiovascular disease in Turkey. Atherosclerosis 156:1–10
Genuth S, Alberti KG, Bennett P et al (2003) Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus: the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26:3160–3167
World Health Organization Consultation (1999) Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. World Health Organization, Geneva (WHO/NCD/NCS/99.2)
Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Can G (2007) Determinants and definition of abdominal obesity as related to risk of diabetes, metabolic syndrome and coronary disease in Turkish men: a prospective cohort study. Atherosclerosis 191:182–190
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Rose GA, Blackburn H, Gillum RF, Prineas RJ (1982) Cardiovascular survey methods, 2nd edn. World Health Organization, Geneva, pp 124–127
Twigg SM, Kamp MC, Davis TM, Neylon EK, Flack JR (2007) Australian Diabetes Society; Australian Diabetes Educators Association. Med J Aust 186:461–465
Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J (2006) Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29:1909–1914
Festa A, Hanley AJ, Tracy RP, D’Agostino R Jr, Haffner SM (2003) Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation 108:1822–1830
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR et al (2010) Diabetes mellitus, fasting glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222
Aso Y, Inukai T, Tayama K, Takemura Y (2000) Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol 37:87–92
Kontush A, Chapman MJ (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58:342–374
Faerch K, Borch-Johnsen K, Holst JJ, Vaag A (2009) Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 52:1714–1723
Ridker PM, Buring JE, Rifai N, Cook NR (2007) Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297:611–619
Qiao Q, Pyörala K, Pyörala M, Nissinen A, Lindström J, Tilvis R, Tuomilehto J (2002) Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 23:1267–1275
Waterworth D, Ribalta J, Nicaud V, Dallongeville J, Humphries SE, Talmud P (1999) ApoCIII gene variants modulate postprandial response to both glucose and fat tolerance tests. Circulation 99:1872–1877
Acknowledgments
Various pharmaceutical companies in Istanbul, Turkey, which have supported the Turkish Adult Risk Factor study, are gratefully acknowledged. We appreciate the dedicated work of the co-workers in the survey teams.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onat, A., Can, G., Çiçek, G. et al. Fasting, non-fasting glucose and HDL dysfunction in risk of pre-diabetes, diabetes, and coronary disease in non-diabetic adults. Acta Diabetol 50, 519–528 (2013). https://doi.org/10.1007/s00592-011-0313-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-011-0313-x