Abstract
Glycated albumin (GA) is recognized as a reliable marker for short-term glycemic monitoring in diabetic patients. We investigated the clinical relevance of GA and the ratio of GA to glycated hemoglobin (A1c) in Korean type 2 diabetic patients. In this retrospective study, we compared A1c, GA, and the GA/A1c ratio and analyzed the relationship between glycemic indices and various parameters in 1,038 Korean type 2 diabetic patients. The patients were divided into two groups: a stably maintained A1c group whose A1c levels did not fluctuate by more than 0.5% for at least 6 months and an unstably maintained A1c group whose A1c levels fluctuated by more than 0.5%. Serum GA was strongly correlated with A1c in both groups. Fasting plasma glucose and postprandial glucose were correlated with GA in unstably maintained A1c group, whereas they were correlated with A1c in stably maintained A1c group. The GA/A1c ratio tended to increase as A1c increased. Postprandial glucose and body mass index affected the GA/A1c ratio. Our data show that serum GA may be a more useful glycation index than A1c for monitoring glycemic control in type 2 diabetic patients with fluctuating and poorly controlled glycemic excursions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The results of the United Kingdom Prospective Diabetes Study (UKPDS) and its follow-up studies demonstrated that intensive glycemic control reduced or delayed hyperglycemia-induced vascular complications in newly onset type 2 diabetic patients [1, 2]. In addition, the duration and severity of hyperglycemia was strongly associated with complications, such as diabetic nephropathy and cardiomyopathy [3–5].
Glycated hemoglobin (A1c) has been used as the gold standard parameter for monitoring diabetes. An average A1c level is closely associated with the onset or prediction of diabetic micro- or macro-vascular complications. [1, 2, 6, 7] However, the use of A1c as an indicator of glycemic control over a 2- 3-month period does not provide information on earlier changes in glycemic control or on various conditions affecting the lifespan of red blood cells [8, 9].
In this regard, the focus has recently been on the use of glycated albumin (GA) as a glycation indicator over 2–3 weeks for diabetic patients. The use of GA as glycemic indicator or the relationship between levels of A1c and GA has been reported already [10, 11]; however, clinical implications of GA and the clinical value of GA compared to Alc in type 2 diabetic patients have not been well elucidated in Korea. We therefore investigated the clinical relevance of GA and the relationship between GA and A1c by measuring GA-to-A1c ratio (GA/A1c ratio) in Korean type 2 diabetic patients.
Materials and methods
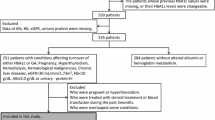
In this clinic-based, retrospective study of type 2 diabetic patients, we analyzed patients who satisfied the following criteria based on their medical records. Inclusion criteria were patients who were enrolled in the diabetes registry of Severance Hospital and those who had been tested for A1c, GA, and fasting/postprandial plasma glucose (FPG/PPG) every 3 months on ≥2 occasions. Exclusion criteria included severe liver or kidney diseases, thyroid disorders, pregnancy, steroid therapy, nephrotic syndrome, and hematological and malignant diseases. The study protocol was approved by the ethics committee of the Yonsei University College of Medicine. A total of 1,038 patients satisfied the criteria, and they were classified into two groups according to A1c fluctuation (Group I, stably maintained A1c group with <0.5% fluctuation in A1c across 6 months; Group II, unstably maintained A1c group with ≥0.5% fluctuation in A1c across 6 months).
Serum GA was determined by an enzymatic method using an albumin-specific proteinase, ketoamine oxidase and albumin assay reagent (LUCICA GA-L, Asahi Kasei Pharma Co., Tokyo, Japan), and a Hitachi 7699 Pmodule autoanalyzer (Hitachi Instruments Service, Tokyo, Japan) [12, 13]. The coefficient of variation (CV) was 1.43%. A1c was measured by high-performance liquid chromatography (HPLC) using a Variant II Turbo (Bio-Rad Laboratories, Hercules, CA). The reference intervals of A1c were between 4.0 and 6.0%, while those of GA were between 11.0 and 16.0%.
Statistical analysis
All data are shown as the mean ± standard deviation. Student’s t-test was used to compare continuous variables between the stably maintained A1c group and unstably maintained A1c group. To evaluate the relationship between A1c and GA levels and other variables, Pearson’s correlation coefficient (R) was used to assess the strength of the relationships between clinical and laboratory variables. Variables with P < 0.05 in univariate analysis were used in the multivariate analysis. Multiple linear regression analysis by stepwise selection was performed using the GA/A1c ratio as a dependent factor. A P-value of < 0.05 was considered significant. The SAS program (version 9.1; SAS Institute, Cary, NC) was used to test the difference between two correlated correlation coefficients. Other statistical analyses were performed using the SPSS package for Windows (version 15.0; SPSS, Chicago, IL).
Results
Baseline characteristics of the patients
Of 1,626 subjects with Korean type 2 diabetes who satisfied the inclusion criteria, 1,038 (561 men and 477 women) patients were finally analyzed in this study. The patients’ demographic and clinical characteristics are summarized in Table 1. The mean age was 59.8 years, and the gender distribution was relatively even. There were no significant differences in age, sex, height, body mass index (BMI), hemoglobin (Hb), total protein, and creatinine between the two groups. The levels of A1c, GA, FPG, and PPG were significantly higher in the unstably maintained A1c group (8.15 ± 1.64, 21.90 ± 8.35%, 7.85 ± 2.87, and 13.14 ± 4.94 mmol/l, respectively) than in the stably maintained A1c group (7.04 ± 0.90, 17.73 ± 4.81%, 6.92 ± 1.74, and 11.33 ± 3.68 mmol/l, respectively) (P < 0.001 for all). Serum albumin levels were different (4.59 ± 0.29 g/dl in Group I vs. 4.50 ± 0.39 g/dl in Group II, P < 0.01). The mean GA/A1c ratio was 2.60 ± 0.54. The GA/A1c ratio of the unstably maintained A1c group was significantly higher than that of the stably maintained A1c group (2.63 ± 0.56 vs. 2.49 ± 0.45, respectively).
Correlation between plasma glucoses levels (fasting and postprandial) and glycation indices (GA and A1c)
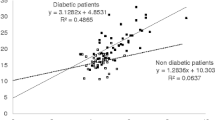
Figure 1 shows the correlation between plasma glucose (FPG and PPG) and glycation indices (GA and A1c). Both FPG and PPG were significantly correlated with GA as well as A1c. In group I, both FPG and PPG were more strongly correlated to A1c levels (r = 0.623, 0.643, respectively, P < 0.001 for both) than to A1c (r = 0.547, 0.524, respectively, P < 0.001 for both). However, in group II, GA showed a higher correlation with FPG and PPG (r = 0.527, 0.640, respectively, P < 0.001 for both) than with A1c (r = 0.502, 0.579, respectively, P < 0.001 for both). In all patients, both FPG and PPG were more strongly correlated to patient’s GA (r = 0.544, 0. 636, respectively, P < 0.001 for both) than to A1c (r = 0.531, 0.6, respectively, P < 0.001 for both).
Correlation between plasma glucose levels (fasting and postprandial) and glycation indices (GA and A1c). a b; Group I (n = 270), c d; Group II (n = 768). Group I, stably maintained A1c group with <0.5% fluctuation in A1c across 6 months; Group II, unstably maintained A1c group with ≥0.5% fluctuation in A1c across 6 months. A1c Glycated hemoglobin, GA Glycated albumin, FPG Fasting plasma glucose, PPG Postprandial plasma glucose
Correlation between serum GA or A1c levels and the GA/A1c ratio
A very strong (at least 0.8) linear relationship was observed between serum GA and GA/A1c ratio in all patients, group I, and group II (r = 0.810, 0.853, and 0.811, respectively, P < 0.001). However, poor (less than 0.3) or fair (0.3–0.5) linear relationship was found in A1c and the GA/A1c ratio in all patients, group I, and group II (r = 0.343, 0.283, and 0.336, respectively, P < 0.01).
Distribution of the GA/A1c ratio and other values according to the A1c levels
The values of GA, FPG, PPG and GA/A1c ratio were analyzed according to A1c levels, which is a well-validated glycation index (Table 2). As expected, plasma glucose levels (FPG and PPG) and GA increased along with A1c levels. The mean GA/A1c ratio ranged between 2.39 and 3.13. The incremental values of GA–A1c were dependent on poor glycemic control.
Linear relationship and simple linear regression model to relate GA with A1c
A very strong (at least 0.8) linear relationship was observed between mean serum GA and mean A1c levels in all patients and group II (r = 0.81 and 0.805, respectively, P < 0.001), and a moderately strong (0.6–0.8) linear relationship was observed in group I (r = 0.733, P < 0.001). Once adjusted for age, sex, BMI, Hb, and albumin, which are known to influence measurement of GA or A1c, the measured Pearson product-moment correlation between serum GA and A1c levels increased to 0.832 in all patients, to 0.771 in Group I and to 0.828 in Group II (P < 0.001 for all).
When GA was assumed to be an independent variable, the simple linear regression model relating GA to A1c became “A1c = 4.14 + 0.18 × GA”. Conversely, when A1c was assumed to be an independent variable, the linear regression model became “GA = −8.01 + 3.66 × A1c”.
Multiple regression analysis of factors for the GA/A1c ratio
We analyzed the factors affecting the GA/A1c ratio. Multiple regression analysis of various clinical and laboratory factors was performed to evaluate their independent association with the GA/A1c ratio in type 2 diabetic patients. In univariate analysis, the GA/A1c ratio was related to age, albumin, Hb, uric acid, cholesterol, triglyceride, FPG, PPG, and BMI. In multiple regression analysis, only PPG (β = 0.052, 95% CI: 0.045–0.06, P < 0.001) and BMI (β = −0.039, 95% CI:−0.049 to −0.029, P < 0.001) significantly influenced the GA/A1c ratio (Data not shown).
Discussion
Accurate determination of glycemic control is of paramount importance in managing diabetes and in improving outcomes and survival of diabetic patients. A1c has been used as the gold standard for monitoring diabetes and as a diagnostic tool [14]. However, there are several problems concerning A1c. Although most studies suggested A1c as a screening tool for diabetes, there is still no consensus on a suitable cutoff values of A1c across different ethnic populations [15]. Furthermore, it is obvious that A1c levels are affected in hematologic disorders shortening erythrocyte lifespan [16–18], such as abnormal iron metabolism [16]. As a result, A1c may be not reliable for monitoring glycemic control under these conditions. GA as a short-term (2–3 weeks) glycation index for diabetes control had been considered as an adjunctive to A1c. Recently, its usefulness has been demonstrated, and it is now gaining popularity as an index of glycemic control during intensive treatment, as a more accurate glycation index for patients with end-stage diabetic nephropathy [19, 20] or gestational diabetes [9, 21] However, clinical implications of GA compared to A1c are not well elucidated for type 2 diabetic patients in Korea.
Based on previous studies on GA [9–11, 19–23], we hypothesized that GA could serve as a better marker of glycemic control than A1c for fluctuating glycemic excursions. We tried to identify (1) which glycation index for diabetes monitoring (A1c or GA) would be more correlated with fasting and postprandial glucose levels and (2) relative clinical implication of GA compared with A1c in Korean type 2 diabetic patients. To address these questions, we analyzed type 2 diabetic patients regardless of glucose control status and classified them into two groups according to A1c fluctuation. Although there was a difference in serum albumin levels (0.09 g/dl) between two groups, the clinical implication of this difference may be negligible. There were no patients with disorders affecting albumin metabolism in this study.
With respect to correlation of glycation index, the results demonstrate two main findings: first, correlation between A1c and GA was very high in patients with type 2 diabetes regardless of glycemic variations. However, correlation between GA and A1c levels was stronger in patients with group 2 than in group 1. Second, in group I, levels of FPG and PPG showed significantly higher correlation with A1c, but in group II, levels of FPG and PPG showed significantly higher correlation with GA (Fig. 1). Although this relationship was demonstrated on a limited number of type 2 diabetic patients, similar result was observed by Paroni R et al. [24]. It suggests that GA may be a more useful glycemic marker than A1c, especially in patients with poor glycemic control.
With respect to relationship between A1c and GA, we adopted the GA/A1c ratio and classified the mean values of GA/A1c ratio according to A1c level. We found the mean GA/A1c ratio had a tendency to increase with higher A1c levels. As shown in Table 2, serum GA level increased by 3–4% for every 1% increase in serum A1c level. Therefore, the GA/A1c ratio increased along with higher A1c may be attributable to more marked increases in GA level than A1c level, especially in patients with poor glycemic control. Koga et al. showed that GA-to-A1C ratio was significantly higher in those treated with insulin than in those treated with diet or oral hypoglycemic agents. HOMA-β showed a significant inverse correlation with GA-to-A1C ratio. This ratio was higher in type 1 diabetic patients than in type 2 diabetic patients [25]. Their result postulated that diabetic patients with decreased insulin secretion, serum GA levels are higher relative to A1c. Based on these result, GA might better reflect postprandial hyperglycemia, which was known to be mainly caused by inadequate or dysfunctional endogenous insulin secretion. Furthermore, we found that the GA/A1c ratio showed significant inverse correlation with BMI, similar to previous reports [23, 25–27]. In contrast, the ratio was positively affected by PPG.
There are some limitations to this study. First of all, this relationship is applicable only to GA measured by enzymatic assay, because there are several methods of GA measurement available and each method provides different reference ranges [9]. Also, its usefulness is more limited to fluctuating and poorly controlled type 2 diabetics, not to all subjects with type 2 diabetes. Finally, there were no analytic results relating the insulin secretory status of diabetes and the usage of glucose-lowering agents (insulin vs. oral glycemic agents or type of oral glycemic agents). However, this study is the first well-designed, large-scale study including more than 1,000 patients that investigated the clinical implications of GA for Korean type 2 diabetics. We suggest that GA measurement might be practically applied as a follow-up monitoring index 3 weeks after intensified antidiabetic treatment to guide the efficacy of intensified regimens in poorly controlled type 2 diabetics.
In conclusion, our data show that serum GA may be a more useful glycation index than A1c for monitoring glycemic control in type 2 diabetic patients with fluctuating and poorly controlled glycemic conditions. Further studies on clinical relevance of glycated albumin on type 2 diabetic patients will make our observations useful for clinical management of these patients.
References
Turner RC, Cull CA, Frighi V, Holman RR (1999) Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK prospective diabetes study (UKPDS) group. JAMA 281(21):2005–2012
Holman R, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589
Atkins RC, Zimmet P (2010) Diabetic kidney disease: act now or pay later. Acta Diabetol 47(1):1–4
Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF (2010) The diabetic cardiomyopathy. Acta Diabetol. doi:10.1007/s00592-010-0180-x
Lim S, Kim DJ, Jeong IK et al (2009) A Nationwide Survey about the current status of glycemic control and complications in diabetic patients in 2006: the committee of the Korean diabetes association on the epidemiology of diabetes mellitus. Korean Diabetes J 33(1):48–57
Diabetes Control and Complications Trial Group (1995) The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44(8):968–983
Gaede P, Vedel P, Larsen N, Jensen GV, Parving H, Pedersen O (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348(5):383–393
Goldstein DE, Little R, Lorenz RA et al (2004) Tests of glycemia in diabetes. Diabetes Care 27(7):1761–1773
Roohk HV, Zaidi AR (2008) A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2(6):1114–1121
Tahara Y (2009) Analysis of the method for conversion between levels of HbA1c and glycated albumin by linear regression analysis using a measurement error model. Diabetes Res Clin Pract 84(3):224–229
Shima K, Abe F, Chikakiyo H, Ito N (1989) The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract 7(4):243–250
Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S (2002) An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 324(1–2):61–71
Kouzuma T, Uemastu Y, Usami T, Imamura S (2004) Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta 346(2):135–143
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69
Hu Y, Liu W, Chen Y et al (2010) Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol 47(3):231–236
Koga M, Saito H, Mukai M, Matsumoto S, Kasayama S (2009) Influence of iron metabolism indices on glycated haemoglobin but not glycated albumin levels in premenopausal women. Acta Diabetol. doi:10.1007/s00592-009-0123-6
Koga M, Murai J, Saito H, Mukai M, Kasayama S (2010) Serum glycated albumin, but not glycated haemoglobin, is low in relation to glycemia in hyperuricemic men. Acta Diabetol 47(2):173–177
Koga M, Saito H, Mukai M, Otsuki M, Kasayama S (2009) Serum glycated albumin levels are influenced by smoking status, independent of plasma glucose levels. Acta Diabetol 46(2):141–144
Inaba M, Okuno S, Kumeda Y et al (2007) Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 18(3):896–903
Peacock TP, Shihabi ZK, Bleyer AJ et al (2008) Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 73(9):1062–1068
Hashimoto K, Noguchi S, Morimoto Y et al (2008) A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care 31(10):1945–1948
Imai T, Oikawa Y, Shimada A (2007) Improved Monitoring of the Hyperglycemic State in Type 1 Diabetes Patients by Use of the Glycoalbumin/HbA1c Ratio. Rev Diabet Stud 4(1):44–48
Takahashi S, Uchino H, Shimizu T et al (2007) Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J 54(1):139–144
Paroni R, Ceriotti F, Galanello R et al (2007) Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem 40(18):1398–1405
Koga M, Murai J, Saito H, Kasayama S (2010) Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 33(2):270–272
Miyashita Y, Nishimura R, Morimoto A, Matsudaira T, Sano H, Tajima N (2007) Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract 78(1):51–55
Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S (2007) Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta 378(1–2):48–52
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, E.Y., Lee, BW., Kim, D. et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 48, 167–172 (2011). https://doi.org/10.1007/s00592-010-0242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-010-0242-0