Abstract
This study was aimed at the assessment of incidence of malignancies in type 2 diabetic patients treated with different sulphonylureas. A matched case–control study was performed. Cases were 195 diabetic patients aged 69.0 ± 9.2 years who had an incident malignancy. Controls were 195 diabetic patients, unaffected by cancer, who were matched with the corresponding case for age, sex, duration of diabetes, BMI, HbA1c, comorbidity, smoking and alcohol abuse. Exposure to hypoglycaemic drugs during the 10 years preceding the event (or matching index date) was assessed. After adjusting for concomitant therapies, exposure to metformin and gliclazide for more than 36 months was associated with a significant reduction in the risk of cancer (adj. ORs with 95% CI: 0.28 (0.13–0.57), p < 0.001, and 0.40 (0.21–0.57), p = 0.004, respectively). Conversely, use of glibenclamide for at least 36 months was associated with increased incidence of malignancies (adj. OR 2.62 (1.26–5.42); p = 0.009). Treatment with insulin, thiazolidinediones, or acarbose, was not associated with significant differences in the incidence of cancer. Long-term treatments with individual sulphonylureas could have differential effects on the risk of cancer. In particular, the possible protective effect of gliclazide, as well as the risk associated with glibenclamide, deserves further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A recent retrospective study has shown that treatment with sulphonylureas could be associated with increased cancer-related mortality, without exploring possible differences among individual sulphonylureas [1]. A preliminary epidemiological report raised the hypothesis that glibenclamide could be associated with a higher mortality for malignancies in comparison with gliclazide [2]. This is in line with experimental data, suggesting differential effects of sulphonylureas on carcinogenesis [3]. The present case–control study was designed to assess the association of malignancies with the use of glibenclamide, gliclazide, and other secretagogues.
Subjects, materials and methods
Data collection
This study was performed on a consecutive series of 1,945 diabetic outpatients, living within the region of Tuscany, and referring for the first time to the Diabetes Outpatient Clinic of the Geriatric Unit of the University of Florence between January 1, 1998, and December 31, 2004. Demographic and clinical data, including history of hypoglycaemic medication, self-reported smoking habits and alcohol intake, were collected, as part of the routine clinical assessment. Alcohol consumption of more than two drinks/day was used as a cut-off to define alcohol abuse. At first visit, following a standard procedure of the Clinic, all patients underwent a physical examination, including measurement of weight, height, and blood pressure, following WHO recommendations [4, 5]. A fasting blood sample was used for determining HbA1c (HPLC, Menarini-Diagnostici, Italy; UNL 6.2%) creatinine, total cholesterol, HDL-cholesterol, and triglyceride (all measured with an automated method: Aeroset, Abbott Laboratories). Comorbidity was assessed through the calculation of Charlson’s comorbidity score (CCS), which includes diabetes and its complications, cardiovascular disease, chronic skin ulcers, renal insufficiency, liver diseases, chronic obstructive pulmonary disease, malignancies, arthritis/arthrosis, HIV-infections [6].
Identification of case patients and controls
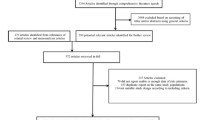
Incident cases of cancer from enrolment up to December 31, 2005, were identified through queries to registers of hospital admissions and causes of death. The event was considered as the first of either hospital admission or death with the international classification of diseases (ICD)-9 codes 140–209. Information on hospital admission was obtained through the Regional Hospital Discharge system, which contains ICD codes of current diagnoses. Deaths from cancer were obtained from the Mortality Registry of Tuscany. Patients with known malignancies at first visit (n = 26) were excluded. Case finding was therefore performed on 1,919 patients (827 and 1,092 women and men, respectively), aged 63.9 ± 12.8 years, with a mean duration of diabetes of 10.7 ± 10.5 years.
Incident cases of cancer were compared with an equal number of controls selected from the same cohort. For each case, the first subsequent patient within the same series, of the same sex, age (±2 years), duration of diabetes (±2 years), HbA1c (±1%), BMI (±2 kg/m2), comorbidity score (CCS ± 1 point), smoking status (current/former/never smoker), and alcohol abuse (yes/no), was taken as control.
Exposure of hypoglycaemic drugs
Exposure to hypoglycaemic drugs during the 10 years preceding the event, in cases, and before the matching index dates, in controls, was assessed. Such assessment was not performed in the rest of the cohort. Drug exposure was obtained from clinical records of the Outpatient Clinic. These records contain self-reported history of hypoglycaemic treatment before the first contact with the Clinic, and all drug prescriptions during follow-up. If the last available visit occurred more than 3 months before the event (or the matching index date), a telephone contact with the patients or their relatives was attempted, in order to collect further information on subsequent drug use. If no such information was obtained, the patient was assumed to have continued the last available hypoglycaemic therapy. Patients were categorized according to their exposure to each agent (any exposure, or treatment for at least 12 or 36 months). Exposure to different combinations of hypoglycaemic treatments were also assessed.
Statistical analysis
Unpaired Student’s t test and Mann–Whitney test were used to compare continuous variables whenever appropriate. Chi-square test was used for between-group comparisons of categorical variables, computing odds ratios (ORs) with 95% confidence interval (CI). Exposure to each drug was compared to lack of exposure to the same drug. Conditional logistic regression was used for multivariate analysis, in order to adjust for concomitant hypoglycaemic treatments; no other adjustments were made, as cases and controls had been carefully matched for the other potential confounders, as described above. Considering that, due to the availability of fixed-dose combinations, glibenclamide is more often associated to biguanides than other secretagogues [7], additional analyses including exposure to combined secretagogues-biguanide therapy among confounders were performed in order to explore interactions. On the other hand, other combinations of hypoglycaemic drugs, less frequent in the sample, were not analysed separately because of the insufficient sample size. Furthermore, although a possible effect on the incidence of cancer of ACE-inhibitors and calcium channel blockers [8] was not confirmed by other studies [9, 10], a further multivariate analysis on 36-month exposure to hypoglycaemic drugs was performed including among covariates’ treatment with those two categories of antihypertensive medications at baseline. All analyses were carried out with SPSS 12.0.1 statistical package, and a p < 0.05 was considered statistically significant.
Results
Mean duration of follow-up in the reference cohort was 6.5 ± 3.8 years, during which 195 new cases of cancer were identified, with an incidence of 2.49/100 persons × year. The most frequent malignancies observed were of gastrointestinal tract (N = 48; of those, 4 oesophageal, 15 gastric, 21 intestinal), female genital tract/mammary glands (N = 27; of those, 6 uterine, 7 cervical, and 12 mammary glands), male genital tract (N = 26; of those, 18 prostate), pancreas (N = 22), and lung (N = 18).
The characteristics of cases and controls, at the time of their first visit at the Outpatient Clinic, are summarized in Table 1. No significant difference was observed between the two groups, except for lower total cholesterol levels in cases.
Follow-up obtained from clinical records was incomplete in 28 (14.4%) cases and 31 (15.9%) controls. Information about treatment was collected through a telephone contact in most of those subjects. In eight (4.1%) cases and six (3.1%) controls, hypoglycaemic treatment after the last contact with the clinic could not be assessed; those patients were assumed to have continued the last available hypoglycaemic therapy.
When considering hypoglycaemic treatments during the previous 10 years, a lower proportion of cases had been exposed to metformin for more than 12 or 36 months in comparison with controls; furthermore, any exposure to gliclazide was more frequent in controls than in cases (Table 2).
Logistic regression model
Insulin secretagogues were often associated with biguanides. Among patients treated with insulin secretagogues for at least 36 months, 89.6, 80.0, 22.2, and 11.3% on glibenclamide, repaglinide, glimepiride, and gliclazide were receiving also a biguanide for the entire duration of secretagogue treatment; all patients treated with chlorpropamide were on combined therapy. A multivariate analysis was therefore performed in order to assess the effect of each treatment after adjusting for concomitant hypoglycaemic medications.
At multivariate analysis, treatment for at least 36 months with insulin sensitizers, insulin secretagogues, insulin, or acarbose, was not associated with significant differences in the risk of malignancies (adj. ORs 0.72 (0.43–1.19), 0.85 (0.52–1.39), 1.01(0.64–1.59), and 0.65 (0.11–3.95), respectively). The results of an alternative model of multivariate analysis, in which metformin and thiazolidinediones were considered separately, and glibenclamide and gliclazide were included as individual drugs, are summarized in Fig. 1. After adjusting for other hypoglycaemic treatments, exposure to metformin for more than 12 or 36 months was associated with a significant reduction of risk (both p < 0.001), while no effect was observed for thiazolidinediones, insulin or acarbose. Among insulin secretagogues, 12- and 36-month exposure to glibenclamide was associated with an increased risk of cancer (p = 0.01 and 0.009, respectively), while gliclazide was associated with a reduction of risk (p = 0.004 and 0.001 for 12-and 36-month exposure, respectively). Similar results were obtained when the exposure for more than 12 and 36 months to the combination of insulin secretagogues and biguanides was added to the model (data not shown).
A further analysis was performed, including treatment with calcium channel blockers and ACE-inhibitors at baseline among covariates, exploring 36-month exposure to hypoglycaemic agents as a possible determinant of incident cancer; the results were not different from those reported above (data not shown).
Among cases who had received glibenclamide for more than 36 months (N = 68), the most frequent forms of cancer were of gastrointestinal (N = 18), female genital tract/mammary glands (N = 13), male genital tract (N = 11), pulmonary (N = 7), and pancreatic (N = 4). After adjustment for biguanide therapy, HRs of exposure to glibenclamide for more than 36 months were 2.45 (0.86–6.94) (p = 0.09), 3.47 (0.95–12.70) (p = 0.06), and 2.12 (0.57–7.92) (p = 0.26) for malignancies of gastrointestinal tract, female genital tract-mammary glands, and male genital tract, respectively. Among cases who had received gliclazide for more than 36 months (N = 19), the most frequent forms of cancer were of gastrointestinal (N = 5), female genital tract/mammary glands (N = 4), and pancreatic (N = 3).
Discussion
The present study does not support the hypothesis that a class effect of sulphonylureas determined an increased risk of cancer, as suggested by a previous study [1]. The design of that study, showing a higher cancer-related mortality in diabetic patients treated with sulphonylureas in comparison with those receiving metformin, did not allow to discriminate whether this difference was due to a risk induced by sulphonylureas, or to a protective effect of metformin [11], or both; similar considerations should be made with respect to another study, which associated metformin with reduced risk of cancer [11]. With respect to sulphonylureas, available data analysed the effect on incidence of malignancies and cancer-related mortality of the class as a whole [1, 11]. We have observed some differences in mortality among individual insulin secretagogues [2, 12], which could be due, at least partly, to differences in cancer-related mortality [2], which are consistent with the results of the present study. This is the first report of a reduction of the risk for cancer in patients treated with gliclazide. The mechanisms underlying this possible protective effect are speculative; however, they could include the well-known anti-oxidant effects of this drug, which has been shown to prevent DNA damage in vitro [3].
We also observed an association between treatment with glibenclamide and the incidence of malignancies. This association was evident only at multivariate analysis; in fact, the concomitant protective effect of metformin, which was co-administered with glibenclamide in most cases, masked the possible increase of risk associated with this sulfonylurea. No increase of risk is observed with insulin secretagogues different from glibenclamide, suggesting a drug-specific effect, which deserves further investigation.
The previously reported association of insulin therapy with increased cancer-related mortality [1] was not confirmed by our data. It should be considered that the described association of insulin with cancer-related mortality could have been determined by some unexplored prescription bias: patients receiving a prescription of insulin are likely to have a more “severe” form of type 2 diabetes, a greater duration of diabetes, or comorbidities contraindicating treatment with oral agents. In fact, the issue of an effect of hypoglycaemic treatments on the risk of cancer has been raised by observational studies, which can be biased by uncontrolled confounders, such as those cited above. In the present study, the match of cases and controls for comorbidity could have eliminated the association of malignancies with insulin treatment. Prolonged exposure to metformin was associated with a reduction of the incidence of malignancies. This is consistent with a previous case-control study [7] showing a protective effect of metformin. The use of thiazolidinediones, which have been recently associated with increased cancer risk in a smaller cross-sectional study [13], was not wide enough to allow any specific statistical analysis. Interestingly, a recent meta-analysis did not show any increase of incident malignancies with rosiglitazone [14].
The main weakness of this study is the fact that information on treatments was obtained partly through prescriptions contained in patients’ clinical records, and partly through self-reported drug history. A gap between prescribed and actual therapy, which could be different with individual agents, could bias the results. Furthermore, it is possible that self-reporting was inaccurate in some cases. However, these biases could hardly explain the differences in risk associated with similar treatments, such as glibenclamide and other insulin secretagogues. It should also be considered that a delay in the identification of incident malignancies could have occurred in some cases. On the other hand, some strengths of this case–control study should also be recognized. The use of a clinical series of patients allowed a very exact match between cases and controls, thus eliminating some confounders usually affecting observational studies, such as body mass index, duration of diabetes, degree of glycaemic control, comorbidities, and smoking and drinking habits. Furthermore, exposure to hypoglycaemic agents was assessed for a time span consistent with the aims of the study.
The limited size of the sample does not allow drawing any definitive conclusion on the association of hypoglycaemic treatments with site-specific forms of cancer. Such information can only be obtained through the analysis of much larger cohorts. On the other hand, it is practically difficult to obtain a detailed clinical characterization of a very large cohort of individuals, making such a study difficult to realize.
Despite the accurate matching of cases and controls, and the adjustment for identified confounders, a prescription bias is inevitable in observational studies on the long-term effects of drug treatments. In fact, it is possible that patients receiving different drugs, although of the same class, are different for some features which were not taken into account in the present study. Results of randomized clinical trials, if available, would be free of this bias; however, the few published long-term studies on sulphonylureas [15, 16] were performed in newly-diagnosed patients, in an age range in which the incidence of malignancies is relatively low. In particular, the UK Prospective Diabetes Study did not report detailed data on incident malignancies [14]. On the other hand, newly-diagnosed cases of cancer in the ADOPT study [15] could be retrieved from the GSK-website, which reports all adverse events. Based on those data, the incidence of cancer was 1.5, 1.1, and 1.1 cases/100 patient × years in the glibenclamide, metformin, and rosiglitazone groups, respectively; the difference did not reach statistical significance due to the relatively small number of cases. Further, ongoing trials [17, 18], could add some relevant information.
In conclusion, glibenclamide, different from other stimulators of insulin secretion, could be associated with increased risk of cancer, while gliclazide could have a protective effect. Further data should be collected, in larger samples of patients, in order to elucidate the actual risk profile of these insulin secretagogues.
References
Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29:254–258
Monami M, Balzi D, Lamanna C et al (2007) Are sulphonylureas all the same? A cohort study on cardiovascular and cancer-related mortality. Diabetes Metab Res Rev 23:479–484
Sliwinska A, Blasiak J, Drzewoski J (2006) Effect of gliclazide on DNA damage in human peripheral blood lymphocytes and insulinoma mouse cells. Chem Biol Interact 162:259–267
WHO (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech.Rep.Ser. 894:i-253
Chalmers J, MacMahon S, Mancia G et al (1999) World Health Organization-International Society of Hypertension Guidelines for the management of hypertension: guidelines sub-committee of the World Health Organization. Clin Exp Hypertens 21:1009–1060
Charlson M, Szatrowski T, Peterson T, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251
Mannucci E, Monami M, Masotti G, Marchionni N (2004) All-cause mortality in diabetic patients treated with combinations of sulfonylureas and biguanides. Diabetes Metab Res Rev 20:44–47
Pahor M, Guralnik JM, Ferrucci L, Corti MC, Salive ME, Cerhan JR et al (1996) Calcium-channel blockade and incidence of cancer in aged populations. Lancet 348:493–497
Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, Warshauer ME, Shapiro S (1998) Calcium channel blockers and the risk of cancer. JAMA 279:1000–1004
Friis S, Sørensen HT, Mellemkjaer L, McLaughlin JK, Nielsen GL, Blot WJ, Olsen JH (2001) Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer 92:2462–2470
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330:1304–1305
Monami M, Luzzi C, Lamanna C et al (2006) Three-year mortality in diabetic patients treated with different combinations of insulin secretagogues and metformin. Diabetes Metab Res Rev 22:477–482
Ramos-Nino ME, Maclean CD, Littenberg B (2007) Association between cancer prevalence and use of thiazolidinediones (TZDs): results from the vermont diabetes information system. BMC Med 5:17–23
Monami M, Lamanna C, Marchionni N, Mannucci E (2008) Rosiglitazone and risk of cancer: a meta-analysis of randomized clinical trials. Diabetes Care Epub ahead of print
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Home PD, Pocock SJ, Beck-Nielsen H et al (2007) Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med 357:28–38
ADVANCE study group (2001) Study rationale and design of ADVANCE: action in diabetes and vascular disease—preterax and diamicron MR controlled evaluation. Diabetologia 44:1118–1120
Conflict of interest statement
Dr. Matteo Monami (MD, PhD) received: (1) speaking fees from Guidotti, Eli Lilly, Merck Sharpe & Dome, Menarini, and Takeda. (2) consultancy fees from Sanofi Aventis and Menarini. Dr. Caterina Lamanna (MD) received: consultancy fees from Merck Sharp & Dome. Dr. Daniela Balzi (MD) has no conflict of interests. Dr. Alberto Marsilii (MD) has no conflict of interests. Prof. Niccolò Marchionni (MD) received: (1) speaking fees from Glaxo Smith and Kline, Guidotti, and Menarini. (2) research grants from Novartis, Novo Nordisk, Sanofi Aventis, Takeda. Dr. Edoardo Mannucci (MD) received: (1) speaking fees from Abiogen Pharma, Glaxo-Smith-Kline, Guidotti, Eli Lilly, Menarini, Merck Sharp & Dome, Merck KgA, Novo Nordisk, Sanofi Aventis, and Takeda. (2) consultancy fees from Sanofi Aventis. (3) research grants from Novartis, Novo Nordisk, Sanofi Aventis, and Takeda. Dr. Mannucci had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monami, M., Lamanna, C., Balzi, D. et al. Sulphonylureas and cancer: a case–control study. Acta Diabetol 46, 279–284 (2009). https://doi.org/10.1007/s00592-008-0083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-008-0083-2